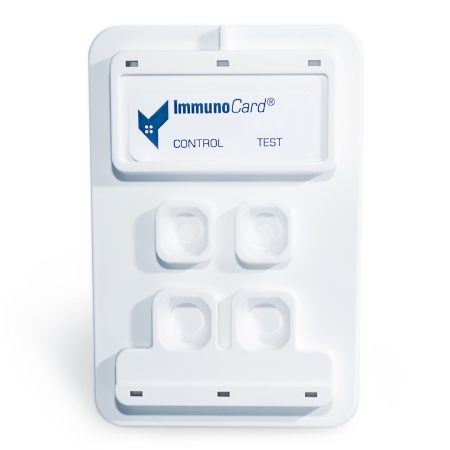
ImmunoCard® Mycoplasma offers a simple 4-step procedure for the detection of active Mycoplasma pneumoniae infection in just 9 minutes
The only rapid EIA for the detection of IgM to Mycoplasma pneumoniae in human serum
Reactive Test Result: Visually detectable BLUE color in BOTH reaction ports
Nonreactive Test Result: Visually detectable BLUE color in CONTROL reaction port (upper left) only
Store the kit at 2-8 C and return the kit promptly to the refrigerator after each use
| Units of Measurement | |
|---|---|
| Manufacturer | MERIDIAN DIAG IN |
| UNSPSC | 41116126 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/img1A4C.PDF |
| MSDS | img1A4C.PDF |