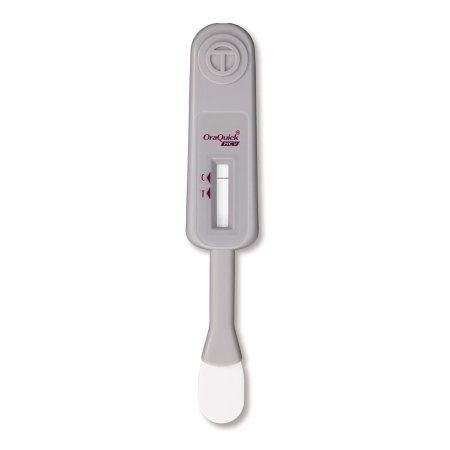
The OraQuick® HCV test is FDA approved for detecting HCV antibodies in fingerstick and venipuncture whole blood
Our simple platform enables healthcare providers to deliver an accurate diagnosis in 20 minutes
The OraQuick HCV Rapid Antibody Test is comprised of both a single-use test device and vial containing a pre-measured amount of a buffered developer solution
The test consists of a sealed pouch with two separate compartments for each component
The developer solution facilitates the capillary flow of the specimen into the device and onto the assay strip
Kit includes: 100 divided pouches each containing a test device, absorbent packet, and developer solution vial (each contains 0.75 mL of a buffered saline solution with an antimicrobial agent); 10 reusable test stands; 100 specimen collection loops; package insert
| Units of Measurement | |
|---|---|
| Manufacturer | ORASURE TECHNOLO |
| UNSPSC | 41116126 |
| Product Specification Sheet | |
| MSDS | MSDS_ORASRE_ORAQUICK_HCV_RAPID_ANTIBODY_TEST_V2.pdf |