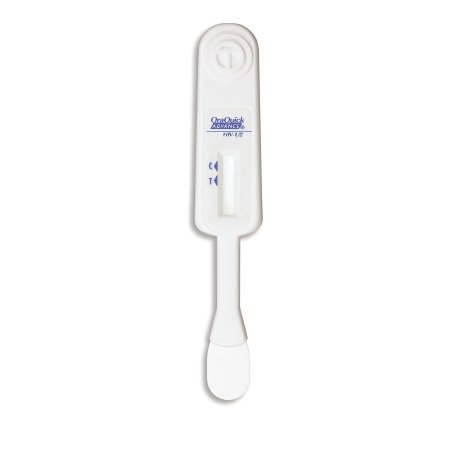
The OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test Kit Controls are quality control reagents for use only with the OraQuick ADVANCE® Rapid HIV-1/2 Antibody Test
Each Kit Control Box contains a package insert and three vials (one HIV-1 positive control, one HIV-2 positive control and one negative control)
Store the controls at 2 to 8 degrees C (35 to 46 degrees F)
One black-capped vial containing 0.2 mL of photochemically inactivated human plasma positive for antibodies to HIV-1, diluted in a defibrinated pool of normal human plasma
One red-capped vial containing 0.2 mL of photochemically inactivated human plasma positive for antibodies to HIV-2, diluted in a defibrinated pool of normal human plasma
One white-capped vial containing 0.2 mL of photochemically inactivated human plasma negative for antibodies to HIV-1 and HIV-2
Negative for Hepatitis B surface antigen and Hepatitis C antibody
| Units of Measurement | |
|---|---|
| Manufacturer | ORASURE TECHNOLO |
| UNSPSC | 41116107 |
| Product Specification Sheet | |
| SDS | SDS_ORASRE_CONTROL_ADVANCE_1NEG_1HIV_1PO_EXEMPTION.pdf |
Related Products
Related products
-
Hcg - Visually Read
TEST KIT, PREGNANCY OSOM (25/KT 18KT/CS) GENZME
This product has multiple variants. The options may be chosen on the product page