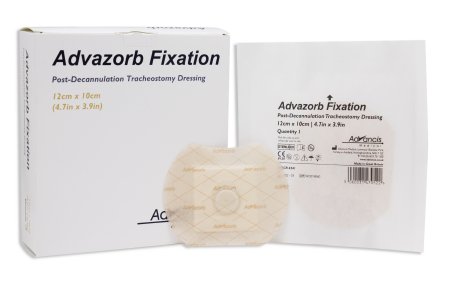
Advazorb Fixation is a post decannulation tracheostomy dressing fitted with a central location button
It is a low profile, non-bulky, skin friendly, hydrophilic foam dressing with a soft silicone contact layer and surrounding adhesive
The silicone adhesive combines security with pain-free dressing changes and has large pores which enable the passage moisture into the absorbent foam while protecting the healing stoma site; this combination ensures the dressing stays comfortably in place while minimizing peristomal irritation
The waterproof backing enables the patient to shower without the need to replace or cover the dressing
The central location button prevents unwanted escape of air when gentle pressure is applied during talking, laughing, coughing and sneezing
Allows atraumatic dressing change
Reduces medical adhesive-related skin injury (MARSI)
Ease of use creates consistency upon application
Provides a barrier for bacteria
Tan
| Units of Measurement | |
|---|---|
| Manufacturer | MEDIUSA |
| UNSPSC | 42311500 |
| Product Specification Sheet |
Related Products
Related products
-
Guaiac
TEST KIT, HEMOCCULT SNG 2HOLE (100/BX) COULTR
This product has multiple variants. The options may be chosen on the product page -
Ifobt
DEVELOPER, FECAL OCCULT TEST BLOOD SOLUTION ER 10ML (8/BX)
This product has multiple variants. The options may be chosen on the product page