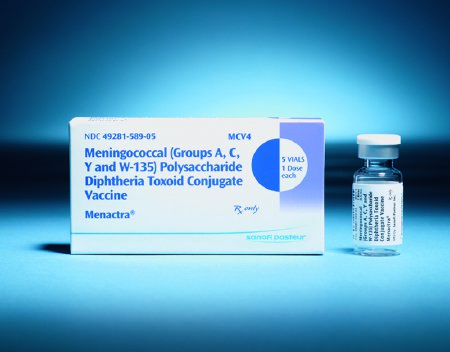
MENACTRA VACCINE, VL 4MCG/.05ML (5/CT)
RESTRICTED - NOT ON FORMULARY
Menactra is indicated for active immunization of individuals 2 through 55 years of age for the prevention of invasive meningococcal disease
Menactra vaccine is administered as a 0.5 mL dose by intramuscular injection
In children 9 through 23 months of age, Menactra is given as a 2-dose series three months apart
Do not administer this product intravenously or subcutaneously
Requires refrigeration
| Units of Measurement | |
|---|---|
| Manufacturer | SANOFI PASTEUR |
| UNSPSC | 51201610 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_SANOFI_VAC_NO_MSDS_REQUIRED.pdf |
| SDS | SDS_SANOFI_VAC_NO_MSDS_REQUIRED.pdf |