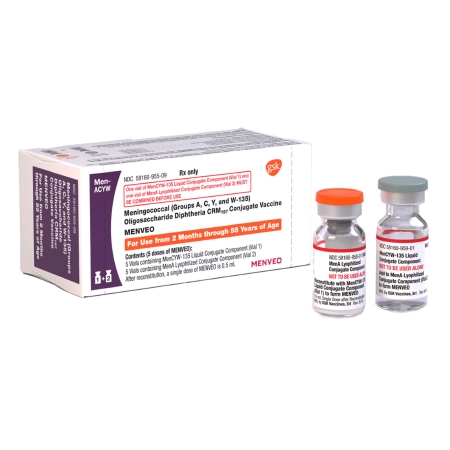
MENVEO VACCINE, SDV 10-5MG/0.5ML (5/CT)
RESTRICTED - NOT ON FORMULARY
MENVEO is a vaccine indicated for active immunization to prevent invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135. MENVEO is approved for use in persons 2 months through 55 years of age
MENVEO is supplied in two vials that must be combined prior to administration: reconstitute the MenA lyophilized conjugate vaccine component with the MenCYW-135 liquid conjugate vaccine component immediately before administration
| Units of Measurement | |
|---|---|
| Manufacturer | GLAXOSMITHKLINE |
| UNSPSC | 51201610 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_SMKLPH_MENVEO_VACCINE_SDV_5CT.pdf |
| SDS | SDS_SMKLPH_MENVEO_VACCINE_SDV_5CT.pdf |