sunmark® clearlax® Powder Unflavored
17.9 oz.
Active Ingredient in Each Dose: Polyethylene Glycol 3350 17 g (Cap Filled to Top Rim).
Relieves occasional constipation (irregularity).
Generally produces a bowel movement in 1 to 3 days.
Increases the frequency of bowel movements and softens the stool.
Grit free.
Dissolves in any beverage.
Gluten and sugar free.
30 once-daily doses.
| Units of Measurement | |
|---|---|
| Manufacturer | McKesson MedSurg |
| UNSPSC | 51171641 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_MGM99_SM_GERICARE_PHARMA_EXEMPTION.pdf |
| SDS | SDS_MGM99_SM_GERICARE_PHARMA_EXEMPTION.pdf |
Related Products
Related products
-
Otc, Analgesics
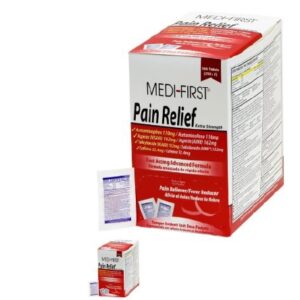
MEDI-FIRST PAIN RELIEF, TAB 162-110-152-32MG (100/BX 24BX/CS
This product has multiple variants. The options may be chosen on the product page -
Otc, Analgesics
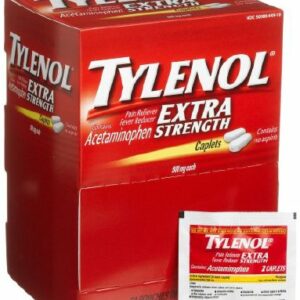
TYLENOL, CAPL 500MG XS 2X50 (50/CT 36CT/CS) J&JOTC
This product has multiple variants. The options may be chosen on the product page