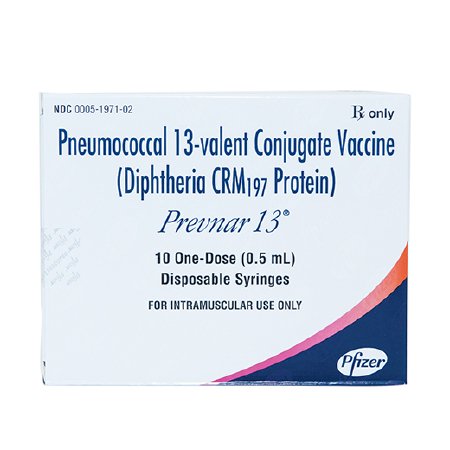
In children 6 weeks through 5 years of age, Prevnar 13 is indicated for active immunization for the prevention of invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F
In children Prevnar is also indicated for active immunization for the prevention of otitis media caused by Streptococcus pneumoniae serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F
No otitis media efficacy data are available for serotypes 1, 3, 5, 6A, 7F, and 19A
In children 6 years through 17 years of age Prevnar 13 is indicated for active immunization for the prevention of invasive disease caused by S. pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F
In adults 50 and older, Prevnar 13 is indicated for the active immunization for the prevention of pneumonia and invasive disease caused by Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F
The tip cap and rubber plunger of the prefilled syringe do not contain latex
Store Refrigerated at 2° to 8°C (36° to 46°F)
| Units of Measurement | |
|---|---|
| Manufacturer | MCKESSON PHARMAC |
| UNSPSC | 51201615 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_9WYETH_PREVNAR_13_SYR1.pdf |
| SDS | SDS_9WYETH_PREVNAR_13_SYR1.pdf |