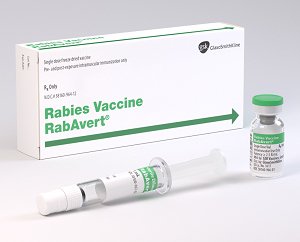
RABAVERT is indicated for preexposure vaccination, in both primary series and booster dose, and for postexposure prophylaxis against rabies in all age groups
The package contains a vial of freeze-dried vaccine, a syringe containing 1 mL of sterile diluent, a sterile needle for reconstitution, and a sterile needle suitable for IM injection
The potency of 1 dose (1.0 mL) of RabAvert is at least 2.5 IU of rabies antigen
WARNING: Cancer and Reproductive Harm – www.P65Warnings.ca.gov
| Units of Measurement | |
|---|---|
| Manufacturer | MCKESSON PHARMAC |
| UNSPSC | 51201617 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_SMKLPH_RABAVERT_KIT_SDV_UNIR_1IU_1ML.pdf |
| SDS | SDS_SMKLPH_RABAVERT_KIT_SDV_UNIR_1IU_1ML.pdf |