Shipments expected to begin around Sept 1, 2021 – Product ships with minimum 30 days dating
BD Triplex assay requires Veritor™ System firmware version 5.5 or greater; firmware version info is displayed on screen when turning on the analyzer or on the orange sticker if it is attached to the analyzer
BD Veritor System for Rapid Detection of SARS-CoV-2 and Flu A+B is For use under an Emergency Use Authorization only: https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/in-vitro-diagnostics-euas-antigen-diagnostic-tests-sars-cov-2thorized for use at the Point of Care (POC), i.e., i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
This test is authorized for use at the Point of Care (POC), i.e., i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditationtories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests
Laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform moderate, high, or waived complexity tests
The test is intended to be used with direct nasal swabs and is not validated for use with swabs in viral transport media
Kit configured for testing anterior nasal swab samples freshly collected, processed, and dispensed directly onto assay test device
Positive results indicate the presence of viral antigens, but clinical correlation with patient history and other diagnostic information is necessary to determine infection status
Positive results do not rule out bacterial infection or co-infection with other viruses
Laboratories within the United States and its territories are required to report all SARS-CoV-2 results to the appropriate public health authorities
- Accessories
- Adaptive Clothing
- Air-Oxygen Masks
- Ancillary Nursing Supplies
- Accessories, Medical Chart
- Applicators, Other Metal/Plastic/Paper S
- Bags, Ice
- Bands/Bracelets, Id Products
- Bath, Sitz
- Bibs, Disposable
- Bins, Storage
- Cotton Rolls
- Cotton/Dacron Swabs
- Cotton/Rayon Balls
- Cover/Lids
- Denture Cups
- Dispensers, Alcohol
- Dressing Jars
- Drinking Cups
- Emesis Bags
- Fire/Rescue Blankets
- Flags Exam Room
- Fracture Bedpans
- Handgrip Covers/Drapes
- Heating Pads, Electric
- Identification Tags
- Labels/Signs
- Leg/Arm Protectors
- Liners – Carafes/Pitchers
- Medical Bags
- Metal Emesis Basins
- Nonmetal Bedpans
- Nonmetal Carafes/Pitchers
- Nonmetal Emesis Basins
- Nonmetal Urinals
- Other Basins
- Other Bowls
- Other Heat/Cold Therapy
- Other Jars
- Other Medical Sundries, Other – Kits/Ute
- Other Medical Sundries, Other Metal/Plas
- Other Medical Sundries, Seals/Ties
- Other Medical Sundries, Utensils/Pt Stay
- Other Swabs
- Other Thermal Products
- Packs, Moist Heat
- Packs/Wraps, Localized
- Patient Belonging
- Personal Belonging Bags
- Proctoscope Swabs
- Racks, Medical Chart
- Solution Bowls
- Sponge Bowls
- Tongue Depressors
- Trays, Food
- Units, Hot Moist Air
- Units, Warming/Cooling, Patient
- Vaginal Swabs
- Wash Basins
- Anesthesia & Suction
- Access-Asprtrs, Comprssr Equip
- Access-Mask,tbng,cnula&mthpc
- Accessories Breathing Circuits
- Accessories, Suction
- Air-Oxygen Masks
- Anesthesia Unit Co2 Absorbants
- Aspirators, Emergency
- Aspirators/Compressors
- Bite Blocks: Surgical Instruments
- Blades, Rigid Laryngoscopes
- Breathing Bags, Respiratory
- Canisters, Collectors, & Canister Sets
- Cannulae, Nasal
- Capnographs Supplies
- Flow Meters & Regulators, Respiratory
- Gas Sampling, Tubing
- Inhalers, Aerosol
- Kits, Custom Suction Canisters & Liners
- Latex Tubing, Med/Surg
- Liners, Suction
- Liquid Oxygen Containers
- Low Volume Aspirators
- Masks/Tubing/Cannulas/Mouthpcs
- Oropharyngeal Airways
- Other Emergency Medical Kits
- Other Impregnated
- Other Tubing, Med/Surg
- Regulators&flowmeters
- Resuscitation, Manual, Respiratory
- Resuscitator Masks
- Suction Canister, Tubing&instr
- Suction, Oral Care
- Tank Cylinders, Carts & Access
- Traps: Respiratory
- Tubing, Suction
- Antibacterials/Anti-Infectives
- Asthma
- Beds And Patient Safety
- Accessories And Parts, Beds
- Accessories, Air Pressure Relief
- Ankle Restraints
- Arm Restraints
- Bed Rails, Beds
- Bed Trays, Beds
- Bedrail Covers
- Beds
- Body Restraints
- Cushions & Positioners
- Electric Beds
- Falls Management Pressure Relief
- Furniture
- Headboards And Footboards, Beds
- Helmets, Protective
- Infant Immobilizers
- Invalid Rings & Cushions
- Lifts And Slings
- Lifts, Lifts/Slings
- Limb Restraints
- Manual Beds
- Mattress Accessories
- Mattress Covers
- Mattress Pads
- Mattresses
- Mattresses/Pads, Stretcher
- Mitts, Restraints
- Non-Powered Pressure Ulcer Mgt (pum)
- Other Cushions
- Other Immobilizers
- Other Patient Restraints & Supports
- Overlays, Pressure Relief (pum)
- Patient Location Alarms
- Patient Room Furniture
- Patient Room Furniture, Furniture
- Patient Room Furniture, Patient Room Fur
- Patient Safety/Fall Management
- Positioning Aids, Patient
- Positioning Aids, Radiographic
- Powered Pressure Ulcer Mgt (pum)
- Retaining Straps, Patient
- Slings, Lifts/Slings
- Supplies, Lifts/Slings
- Vest, Restraints
- Wrist Restraints
- Blood Collection – Containment
- Blood Collection Sets
- Body Care
- Compression
- Containers
- Cpap
- Custom
- Custom & Standard
- Defibrillators
- Diagnostic Cardiology
- Diagnostic Disposables
- Access – Tens Units
- Access-Ekg/Ecg Monitor Electrd
- Accessories, Chargers – Batteries
- Accessories, Ear Irrigation
- Accessories, Oximeters, Respiratory
- Anoscopes
- Batteries – Diag Equipment
- Belts/Straps, Electrode
- Button/Miniature Batteries
- Cables, Ecg Reusable
- Calipers, Ecg
- Covers, Ultrasonic Probe
- Diagnostic Tools, Phys Med/Rehab
- Disposable, Cuffs, Sphyg.
- Distilled/Deionized Water
- Ear Probe Tips
- Ear Specula
- Ecg Recording Paper
- Electrocardiographic Electrodes
- Electrocardiographic Neonatal Electrodes
- Electrode Coupling Gel
- Envelopes Storage, X-Ray Film
- Fetal Heart Monitoring Paper, Ultrasonic
- Filters, Sterilization
- Food
- Gel Warming Units, Electromedical
- Instrument Batteries
- Kits, Ear Irrigation
- Leads Ecg Reusable
- Light Bulbs, Endoscopic
- Lighting Bulbs, Fixtures, Parts
- Lubricating Disposables
- Misc Diag Supplies/Acces
- Mouthpieces/Nose Clips, Respiratory
- Office Supplies
- Oral/Rectal/Aux Elec Therm Probe Covers
- Other Batteries
- Other Disposables, Medicine Cups
- Other Electrode Accessories
- Other Enema Products
- Other Radiological
- Other Recording Paper
- Other Ultrasonic Detectors
- Otoscope Disposables, Light Bulbs, Endos
- Pouches, Telemetry, Electromedical
- Printer Paper
- Pulmonary Spiro Access
- Sheet X-Ray Film
- Specula, Post Surgical Garments
- Tape Measure
- Temporal/Forehead – Contact Probe Covers
- Throat, X-Ray Shield
- Tympanic/Ear Thermometer Probe Covers
- Ultrasonic Coupling Gel
- Vaginal Specula
- View Boxes, X-Ray
- Diagnostic Imaging
- Disposable Underwear
- Durable Medical Equipment
- Adl, Medication Organizers/Dispensers Ad
- Aluminum Canes
- Animal Skin, Pressure Relief
- Bath Mats
- Bathing Adl
- Cane Parts
- Canes
- Commode Chairs
- Covers, Urinal
- Eating And Drinking Adl
- Grooming And Dressing Adl
- Lumbar Cushions
- Other Adl Products
- Other Canes
- Pails, Commode
- Protectors, Heel/Elbow
- Rails/Grab Bars, Ambulatory Aids
- Reaching And Gripping Adl
- Rollators
- Shower/Bath Chairs
- Toilet Aids Adl
- Walker Parts
- Walkers
- Wheelchair Cushions
- Wheelchair Parts
- Wheelchairs
- Wooden Canes
- Electronic Wearables
- Exam & Patient Room Furnishing
- Baby Seats & Strollers
- Bins, Shelving/Parts
- Blood Drawing Chairs
- Cabinets/Wall Desks
- Carts
- Communication Aids/Equipment
- Cubicle Curtains, Reusable
- Enuresis Alarms
- Headlights: Surgical Instruments
- Heel Stirrup Covers
- Infant Blankets
- Instrument/Equipment Carts
- Kick Buckets
- Lift Chairs
- Lighting/Heat Lamps
- Lights, Examination
- Lights, Surgical
- Mayo Stands & Trays
- Other Alarm Products
- Other Cabinets
- Other Carts
- Other Chairs
- Other Equipment, Furniture
- Other Fixtures, Other – Furniture/Equipm
- Parts, Carts
- Penlights
- Printers
- Recliner Chairs
- Screens, Privacy
- Service/Utility Carts
- Stools
- Stretchers
- Stretchers, Stretchers Transfer Backboar
- Stump Socks
- Surgical Room Furn & Equip
- Exam Tables
- Feeding Supplies
- Adapters And Connectors
- Feeding Pump Accessories
- Feeding Pump Backpacks
- Feeding Pump Batteries
- Feeding Pump Repair Parts
- Gastrostomy Tubes
- Gastrostomy Tubes – Full Length
- Gravity Bags
- Jejunal Tubes – Full Length
- Nasogastric Tubes
- Oral Tip Syringes
- Other Enteral Feeding Accessories
- Reusable Irrigating Syringes
- Securement Devices
- Spike Sets
- Tube Decloggers
- Tube Holders
- Fluff Dressings
- Food Service Disposables
- Food Supplements
- Diabetic: Enteral Tube Feeding
- Digestive Aids/Laxatives
- Elemental And Semi-Elemental
- Enteral Tube Feeding
- Enteral Tube Feeding, Nutritional-Condit
- Feed Supplies-Infant Nursing
- Food/Beverage Thickeners
- Immune Enhancing: Enteral Tube Feeding
- Nutrient Dense Supplements: Medical Nutr
- Nutritional-Condition Specific
- Nutritional-Modular Components
- Nutritional-Modular Components, Critical
- Nutritionals – Infant
- Nutritionals – Metabolics
- Nutritionals – Miscellaneous
- Nutritionals – Pediatric
- Nutritionals-Oral Supplements
- Oral Nutritional Supplements: Medical
- Oral Supplements – Diabetic
- Oral Supplements – Wound Care
- Other Enteral Tube Feeding Formulae
- Other Medical Nutritional/Supplements
- Other Supplements, Nutritionals – Miscel
- Pediatric/Infant Formula: Medical Nutrit
- Pediatrics, Nutritionals – Infant
- Pulmonary: Enteral Tube Feeding
- Renal: Enteral Tube Feeding
- Standard: Enteral Tube Feeding
- Total Pediatric: Enteral Tube Feeding
- Vitamins/Supplements
- Wound Healing: Enteral Tube Feeding
- Gauze
- Combine Dressings
- Dressings, Germicidal Dressings
- Dry Nonadherent Dressings
- Eye Patches
- Fluff Dressings
- Nasal Trusses
- Nonsterile Gauze Sponges
- Nonsterile Gauze, Roller Gauze Bandages
- Nonsterile Nonwoven Sponges
- Packers Gauze Bandages
- Sterile Gauze , Roller Gauze Bandages
- Sterile Gauze Sponges
- Sterile Nonwoven Sponges
- Gloves
- Autopsy/Utility/Housekeeping
- Cleanroom Gloves – Other
- Cut Resistant Gloves
- Dispensers, Glove
- Glove Box Holders/Dispensers
- Glove, Exam Latex Nonstr Pf
- Glove, Exam Latex Nonstr Pf W/Emoll
- Glove, Exam Latex Sterile Pf
- Glove, Exam Latexfree Nonstr Pf
- Glove, Exam Nitrile Nonstr Pf
- Glove, Exam Nitrile Nonstr Pf W/Emoll
- Glove, Exam Nitrile Sterile Pf
- Glove, Exam Vinyl Nonstr Pf
- Glove, Exam Vinyl Sterile Pf
- Glove, Surg Latex Pf
- Glove, Surg Latexfree Pf
- Glove, Surg Latexfree Pf W/Emoll
- Gloves Nitrile Clean Room – Sterile
- Other Gloves
- Other Gloves, Finger Cots
- Pressure Gloves
- Utility Gloves
- Hand Hygiene
- Hand Hygiene, Wipes, Personal Care: Skin
- Hand Sani – Alcoh Fr Desp Refill Btl/Bag
- Hand Sani – Alcohol Desp Refill Btl/Bag
- Hand Sani/Soaps- Disp/Brackets/Stands
- Hand Sanitizer – Alcohol Btl/Bag
- Hand Sanitizer – Alcohol Free Btl/Bag
- Hand Sanitizer – Wipes
- Hand Soap/Scrb – Foam Des Refill Btl/Bag
- Hand Soap/Scrb – Liq Desp Btl/Bag Other
- Hand Soap/Scrb – Liq Desp Refill Btl/Bag
- Hand Soaps/Scrubs – Foam Btl/Bag
- Hand Soaps/Scrubs – Waterless No Rinse
- Ip Hygiene Stations And Other
- Other Skin Care Products(dnu)
- Hand Hygiene/Surface Disinfect
- Alcohol
- Alcohol Based Hand Sanitizer
- Alcohol Preps
- Alcohol-Free Hand Sanitizer
- All Other Infection Prevention
- Antiseptic Preps: Skin Care
- Bleaches: Housekeeping
- Chemical Spill Kits
- Cleanroom – Alcohol
- Cleanroom – Sprays/Wet Wipes
- Cleanroom Dry Wipers
- Floor Mats
- Hand Hygiene, Disposable Wash Cloths
- Hand Hygiene, Wipes, Personal Care: Skin
- Hand Sanitizer Dispenser Equipment
- Hand Sanitizer Wipes
- Infectious/Cleanup/Turnover Spill Kits
- Instrument/Surface Disinfectants
- Iodophor Preps: Skin Care
- Iodophors: Skin Care
- Laundry Bags,infection Control
- Liquid Scrubs, Antimicrobial: Skin Care
- Liquid Soaps: Skin Care
- Liquid/Foam Hand Soaps-No Rinse:skin Car
- Other Hazardous Waste Control Products
- Other Housekeeping
- Other Skin Care Products
- Other Spill Kits
- Peroxides
- Reclosable Bags
- Red Bag Infectious Waste Control
- Scrub Sponges, Antimicrobial: Skin Care
- Skin & Surgical Prep, Wipes, Personal Ca
- Skin Prep
- Solidification Treatment, Spill Kits
- Spill Kits
- Surface Disinfectation Wipes
- Surface Disinfection
- Waste Receptacles, Contaminated
- Wipes
- Home Environments
- Housekeeping
- Bags Kick Bucket
- Bags Laundry
- Bags Laundry Biohazard/Ylw
- Bags Laundry Watersoluble
- Bags Misc/Accessories
- Bags Other Chemo Transport
- Bags Paper
- Bags Trash 12-16 Gal
- Bags Trash 20-30 Gal
- Bags Trash 31-35 Gal
- Bags Trash 4-11 Gal
- Bags Trash 40-50 Gal
- Bags Trash 55-65 Gal
- Bags Zipper
- Bags Zipper Amber
- Bio Hazard – Other
- Chemical Bath/Toilet/Urinal
- Chemical Carpet/Stain Remover
- Chemical Cleaners
- Chemical Degreasers
- Chemical Dishwashing
- Chemical Dusting/Polishing
- Chemical Floor Cleaning
- Chemical Floor Finishing
- Chemical Floor Stripping
- Chemical General Cleaning
- Chemical Glass
- Chemical Laundry
- Chemical Misc
- Chemical Odor Control
- Clean Rm Accessories Papr/Pads/Tacky Mat
- Facial Tissue & Dispensing
- Hardware Bottle/Trigger Empty
- Hardware Brooms/Dust Pans
- Hardware Buckets/Accessories
- Hardware Cart Covers
- Hardware Carts
- Hardware Cloth/Sponge/Scrubber
- Hardware Floor Machines/Parts
- Hardware Hampers/Accessories
- Hardware Misc
- Hardware Mops/Squeegees
- Hardware Mops/Squeegees/Mopheads
- Hardware Storage Bin/Organizer
- Hardware Trash Can/Accessories
- Hardware Vacuum/Sweeper/Parts
- Janitorial Supplies
- Kitchen Supplies
- Napkins & Dispensing
- Other Bags
- Paper Prod (towels/Tisues/Tp)
- Reclosable Bags
- Red Bag Inf Waste- Autoclavable
- Red Bag Infectious Waste Control
- Seat Covers & Dispensing
- Toilet Tissue & Dispensing
- Towels & Tissue, Towels Roll & Dispensin
- Towels C/M/S Fold & Dispensing
- Towels C/M/S Fold And Dispensi
- Towels Roll & Dispensing
- Trash Can Liners
- Trash Receptacles/Hampers
- Wipes Dry & Dispensing
- Yellow Bag Infectious Waste Control
- Zipper Lock/Dietary Bags
- Inco Wipes
- Incontinence Briefs
- Interpretive Ecg
- IV Therapy
- Adapters/Connectors, Miscellaneous Urolo
- Adapters/Connectors, Vials, Medicine
- Admin Sets
- Admin Sets, Blood Collection Needle Sets
- Administration Sets, Parenteral
- Ambulatory Infusion Sets, Parenteral
- Arm Board Covers
- Arm Boards & Accessories
- Connector Cap Disinfection, Parenteral
- Connectors & Adapters, Parenteral
- Connectors Needless, Parenteral
- Cstd
- D5w-500ml
- Dispenser IV Fluid, Parenteral
- Immobilizers, IV Catheter/Needle
- Infusion Pump Accessories & Other
- Infusion Pump Asset Return Boxes
- Infusion Pump Batteries
- Infusion Pump Medication Bags
- Infusion Pump Pouches
- Infusion Pump Repair Parts
- Infusion Pump Sets
- Infusion Pump Sets – Lvp
- Intraosseous Infusion Needles
- Intravenous Poles
- IV Fluid Containers, Parenteral
- IV Pumps, Infusion Pumps & Equipment
- Lactated Ringer-500ml
- Medicine Kp&t-Cus
- Multi-Use, Extension Sets
- Other – IV Solutions
- Other Cardiac Catheter Accessories
- Other Emergency Medical Equip/Supplies
- Other IV Therapy, Kits
- Other IV Therapy, N/S Accessories
- Other Parenteral Supplies
- Other Renal Dialysis Supplies
- Other, Other IV Therapy
- Pump Accessories
- Safety IV Catheters
- Security Seals
- Sod. Chl. – Irr
- Standard Huber Needles/Sets
- Standard IV Catheters
- Sterile Water – Irr
- Tourniquet Straps
- Tpn Bags And Sets
- Transfer Device, Transfer/Access Devices
- Winged IV Catheters,safety,parenteral
- Kit/Tray Sml Wound/Lac/Incis/D
- Kit/Tray Sml Wound/Lac/Incis/Dsg
- Kits
- Kits, Custom & Standard
- Cleanup/Turnover Kits Standard
- Custom, Kit Specialty/ Personal/Admiss
- Custom, Kit/Tray Sml Wound/Lac/Incis/Dsg
- Custom, Tray/Kit Anesthesia/Pain
- Custom, Tray/Kit General/Endo/Gi
- Custom, Tray/Kit Ob Gyn Gu
- Custom, Tray/Kit Proc Plastics
- Other Standard Kits & Trays
- Standard, Kit Specialty/ Personal/Admiss
- Standard, Kit/Tray Sml Wound/Lac/Incis/D
- Standard, Tray/Kit General/Endo/Gi
- Standard, Tray/Kit Ob Gyn Gu
- Standard, Tray/Kit, Proc Eent/Ophthalmic
- Standard, Tray/Kit, Proc Ortho/Neuro
- Lab – Cardiac Marker
- Lab – Poc Chemistry
- Lab-Blood Bank
- Lab-Blood Glucose Meters & Sup
- Lab-Chemistry
- Lab-Coagulation
- Lab-General Lab Equipment
- Lab-Hematology
- Lab-Immunoassay
- Lab-Instrument Driven Testing
- A1c Accessories
- A1c Analyzers
- A1c Cals/Ctrls
- A1c Reagents
- Cardiac Marker Reagents
- Hemoglobin Reagents
- Lipid Accessories
- Lipid Reagents
- Pt/Inr Accessories
- Pt/Inr Analyzers
- Pt/Inr Cals/Ctrls
- Pt/Inr Reagents
- Quantitative Glucose Analyzers
- Quantitative Glucose Reagents
- Urinalysis Accessories
- Urinalysis Analyzers
- Urinalysis Analyzers Promo Bundle
- Urinalysis Reagents
- Waived Chemistry Accessories
- Waived Chemistry Reagents
- Waived Hematology Consumables
- Waived Hematology Reagents
- Wbc Reagents
- Lab-Lab Services
- Lab-Lab Supplies
- Lab-Microbiology
- Lab-Molecular
- Lab-Pathology
- Lab-Rapids
- Lab-Respiratory Testing
- Lab-Specimen Collection
- Lab-Urinalysis
- Miscellaneous Respiratory
- Needles & Syringes
- Conventional Hypo Combos
- Conventional Hypo Needles
- Conventional N&s, Conventional Hypo Syri
- Conventional Pen Needles
- Conventional Tb Needle/Syr/Combo
- Dnu / Delete Post Movement Of Items
- Misc / Other, N/S Accessories
- Misc / Other, Pressure Monitoring/Stopco
- N/S Accessories
- Other Syringes – Legend
- Safety Hypo Needle Hinged/Flip
- Safety Hypo Needle/Syr Combo Hinged/Flip
- Safety Hypo Needle/Syr Combo Retractable
- Safety Hypo Needle/Syr Combo Sliding
- Safety Insulin Pen Needle
- Safety Needle Combos – Hinged Pivoting
- Safety Needle Combos – Shielding
- Safety Needle Combos – Sliding Sleeve
- Safety Needles – Hinged/Pivoting
- Safety Syr/Needles – Retracting &combos
- Safety Tb Needle/Syr/Combo
- Specialty Conventional – Tb, Insulin
- Spinal Needles (quincke, Tuohy)
- Syringe 20cc/25cc/30cc/35cc
- Syringe 3cc/5cc/6cc/7cc
- Syringe Bulb/Irrigation/Cath Tip
- Syringe Oral/Enteral/Enfit
- Office Supplies
- All Other Office Supplies, General Offic
- All Other Office Supplies, Industrial
- All Other Office Supplies, Other
- All Other Office Supplies, Technology
- Binders & Chart Holders
- Coloring Books, Stickers, Toys
- Essendant Office Supplies, General Offic
- Essendant Office Supplies, Industrial
- Food Service
- Medical & Patient Forms
- Other Office Supplies
- Standard Batteries
- Orthopedic
- Orthopedics
- Abdominal Binders
- Accessories, Paraffin Baths
- Aluminum Crutches
- Arch Orthoses/Supports
- Arm Slings
- Arm Slings/Elevators/Elbow
- Arm Splints/Supports
- Back, Orthoses/Splints/Supports
- Balance, Exercise
- Balls, Exercise Phys Med/Rehab
- Bands/Putty, Exercise
- Blankets, Circulating Fluid
- Boots, Cast Products
- Cast Protectors, Orthopedic
- Cast Shoes
- Cast Shoes/Post Op Shoes
- Casting Prod(tape/Plast/Pad)
- Cervical Collars Acute Care
- Cervical Collars Extrication
- Cervical Collars Foam
- Cervical Collars Other
- Cervical Collars Rehabilitation
- Cervical Cushions
- Chest Binders
- Clavicle Straps
- Cpm Lower Limb Exercise
- Crutch Parts
- Crutches
- Elbow Immobilizers
- Elbow, Splints/Supports
- Electric Cast Cutters
- Electroanalgesic Units, Tens
- Electroanalgesic, Tens Electrodes
- Finger Splints
- Foot/Ankle, Orthoses/Splints/Supports
- Hand, Splints/Supports
- Hba Products
- Heel Liners
- Heel Stirrups: Orthopedic
- Hernia Trusses
- Hip, Orthoses/Supports
- Hot Water Bags/Bottles
- Insole Liners
- Knee Immobilizers
- Knee, Orthoses/Splints/Supports
- Leg, Orthoses/Splints/Supports
- Lower, Extremity Exercisers
- Lumbosacral Supports
- Massagers, Physical Medicine/Rehab
- Mats, Exercisers
- Moleskins, Pressure Relief
- Neuromuscular Stimulator Electrodes
- Orthopedic Shoes
- Orthotic/Shoe Inserts
- Other Cast Products: Orthopedic
- Other Crutches
- Other Orthopedics Supplies, Contoured Su
- Other Orthopedics Supplies, Finger Cots
- Other Orthopedics Supplies, General Immo
- Other Orthoses/Splints/Supports
- Other Physical Medicine/Rehabilitation
- Other Physical Therapy
- Other Soft Goods
- Other Traction Supplies
- Other, Other Orthopedics Supplies
- Padding, Cast Products
- Pads, Circulating Fluid
- Parallel Bars: Phys Med/Rehab
- Physical Therapy, Physical Therapy
- Plaster, Cast Products
- Pneumatic Splints/Supports
- Protectors, Finger
- Pumps, Circulating Fluid
- Shoulder Immobilizers
- Shoulder, Orthoses/Splints/Supports
- Sleeves/Protectors, Pressure Relief
- Soft Goods, Other Pressure Relief
- Soft Goods, Overlays, Pressure Relief (p
- Soft Goods, Rib Belts/Abdominal/Back Sup
- Soft Goods, Wrist/Forearm/Finger Splints
- Spine Boards
- Splinting Devices: Orthopedic
- Stairs, Exercisers
- Stockinettes
- Supplies, Exercisers
- Suspensories/Trusses
- Synthetic, Cast Products
- Systems, Traction
- Tens Cables/Leads
- Toe, Splints/Supports
- Transfer Boards, Beds
- Trapeze Exercisers
- Ultrasound Units: Phys Med/Rehab
- Units, Paraffin Baths
- Upper, Extremity Exercisers
- Walking Heels: Orthopedic
- Water Cushion, Pressure Relief
- Weights, Exercisers
- Wooden Crutches
- Wrist Immobilizers
- Wrist,orthoses/Splints/Supports
- Ostomy
- Adult Bags W/O Anti-Reflux Valve Urinary
- Appliance Belts, Ostomy
- Bile Collection Bags
- Collection Bags, Ostomy
- Fecal Units
- Ileostomy Appliance Kits
- Inserts, Ostomy
- Irrigators, Ostomy
- Mini Ostomy Pouches
- Odor Control Products, Ostomy
- One Piece Ostomy System, Closed
- One Piece Ostomy System, Drainable
- One Piece, Urostomy
- Ostomy Supplies
- Other, Urostomy
- Pastes & Powders: Ostomy
- Skin Barriers, Wafers: Ostomy
- Sleeves, Ostomy Irrigators
- Stoma Caps, Ostomy
- Tail Closures, Ostomy
- Two Piece Ostomy System, Closed
- Two Piece Ostomy System, Drainable
- Two Piece, Urostomy
- Wafers W/Flange: Ostomy
- Other Sizes
- Patient Assessment/Monitoring
- Accessories, Sphyg.
- Audiometers
- Audiomtry/Otoscope/Ophthalmic
- Baby Analog Scales
- Baby Digital Scales
- Balances, Electronic
- Battery Operated Endoscopic Power
- Calibrators, Spirometer/Pneumotachometer
- Chair Scales
- Computer Parts
- Diagnostic Spirometers
- Disposable Forehead Thermometer Strips
- Electronic Stethoscopes
- Electronic, Units, Sphyg.
- Eye, Color Discrimination Charts
- Eye, Visual Acuity Charts
- Floor Analog Scales
- Floor Digital Scales
- Glass Patient Thermometers
- Handles, Rigid Laryngoscopes
- Inflation Sets, Sphyg.
- Line Operated Endoscopic Power
- Manual Blood Pressure
- Manual, Units, Sphyg.
- Miscellaneous Electromedical Supplies
- Monitors, Auto Bp
- Ophthalmo/Otoscope Sets
- Ophthalmoscopes
- Oral/Rectal/Aux Dig Stick Thermometers
- Oral/Rectal/Aux Disposable Thermometers
- Oral/Rectal/Aux Elec Therm With Probe
- Other – Diag Supplies/Access
- Other Auditory
- Other Cables & Leads
- Other Ophthalmic
- Other, Scales
- Otoscopes
- Parts/Accessories Scales
- Physiologic Monitoring Systems
- Probes, Oximeter, Respiratory
- Pulse Oximeter, Respiratory
- Retinoscopes
- Reusable, Cuffs, Sphyg.
- Scales / Height Rods, Tape Measure
- Specialty Patient Thermometers
- Standard Stethoscopes
- Stethoscope Parts
- Temporal Thermometer (contact)
- Temporal Thermometer (non-Contact)
- Thermometer Replacement Parts
- Tonometers: Ophthalmological
- Tympanic/Ear Thermometers
- Visual Function Analyzers
- Wheelchair Scales
- Personal Care
- Body Care, Personal Care – Patient
- Body Care, Shamp/Conditnr/Bdy Wsh/Bth Kt
- Body Care, Wipes Dry & Dispensing
- Bodywash_soap
- Bodywash_soap No Rinse
- Breast Pumps & Accessories
- Condoms
- Dental Supplies
- Denture Adhesive
- Denture Cleaner
- Deodorants_antiperspirants
- Face_lips_ears
- Feminine Hygiene
- Flossing
- Freshener
- Hair Care
- Hair Combs_brushes
- Hair Shampoo_conditioner
- Hair Shampoo_conditioner No Rinse
- Maternity
- Mouthwash
- Nail Care
- Nail Clippers
- Oral Care, Personal Care – Patient
- Personal Care-Feminine Hygiene
- Prsnl Care-Nursng Pd/Brst Pump
- Sanitary/Ob Pads
- Shaving Cream
- Shaving Razors
- Shaving Skin Conditioner
- Shaving, Shaving Skin Conditioner
- Suction
- Swab_toothette
- Tampons
- Toothbrushes
- Toothpaste
- Personal Care – Patient
- Personal Protective Equipment
- Accessories, Respirator Masks
- Cleanroom Frocks, Sleeves And Other
- Cleanroom Gown Sterile
- Cleanroom Headwear/Hoods
- Cleanroom Shoecovers/Boots
- Coveralls, Staff
- Coveralls, Staff – Cleanroom
- Ear Plugs
- Eyeglasses
- Face Shields & Accessories
- Gowns Chemo
- Head Coverings, Surgical
- Isolation Gowns, Reusable, Staff
- Isolation/Procedure Gowns (aami 2 Level)
- Isolation/Procedure Gowns (aami 3 Level)
- Isolation/Procedure Gowns (aami 4 Level)
- Isolation/Procedure Gowns (aami I Level)
- Isolation/Procedure Gowns (fluid Resist)
- Isolation/Procedure Gowns (not Rated)
- Lab Coats, Disposable,staff
- Lab Coats, Reusable, Staff
- N95 Mask Accessories
- N95 Medical Masks
- N95 Or Greater Filter Industrial Mask
- Other – Apparel/Personal Prot
- Other Safety Products
- Papr & Accessories
- Patient Gowns Pediatric Disposable
- Patient/Consumer Protective Face Masks
- Patient/Exam Gowns Disposable
- Patient/Exam Gowns Reusable
- Pediatric Patient Gowns Reusable
- Procedure Mask (astm Level 1)
- Procedure Mask (astm Level 2)
- Procedure Mask (astm Level 3)
- Procedure Mask (not Rated)
- Respirator Mask, Gas Masks & Accessories
- Safety Glasses & Accessories
- Safety Goggles
- Scrub Suits, Disposable
- Scrub Suits, Reusable
- Shoe Covers, Surgical
- Staff Disposable
- Staff Ears, Ear Plugs
- Staff Eyes, Facial And Eye Protection
- Surgical Mask (astm Level 1)
- Surgical Mask (astm Level 2)
- Surgical Mask (astm Level 3)
- Surgical Mask (not Rated)
- Pharmaceutical Feed
- Pharmaceutical Product
- Procedure Equipment (sterilize
- Respiratory Therapy
- Accessories/Parts Allied Healthcare Prod
- Adapters & Connectors, Respiratory
- Aerosol / Nebulizer Masks
- Aerosol / Nebulizer Tubing
- Bronchial/Exerciser Spirometers
- Brushes, Tracheal Tubes
- Buttons, Tracheostomy (plugs)
- Connector
- Filters, Respiratory
- Heat/Moisture Exchange
- Holders, Endotracheal Tubes
- Holders, Tracheostomy Tubes
- Humidifiers
- Inner Cannula, Tracheostomy
- Misc Humidification
- Misc Trach
- Miscellaneous Respiratory
- Mouth-To-Mask Cpr Resuscitation Masks
- Nasal Aspirators
- Nebulizer Access: Mouthpiece/Tee/Tubes
- Nebulizer Compressor (equipment)
- Nebulizer Kits, Handheld (no Equipment)
- Nebulizer Parts: Repair/Replac/Filters
- Other Respiratory, Adapter/Connectors
- Oxyg Concentrator/Compressor Accessories
- Oxyg Concentrator/Compressor Units
- Oxygen Concentrator Filters
- Oxygen Connecting Tubing
- Straps, Tracheal (twill Tape)
- Stylets, Tracheal
- Suction Catheters Trays Closed System
- Suction Catheters Trays Open System
- Suction Catheters With Valve (single)
- Surgical Aspirators
- Trach Cleaning Kits
- Valves, Tracheostomy (speaking)
- Vent Accessories & Other
- Vent Filters
- Vent Repair Parts
- Rig Components
- Rigs
- Rx – Asthma
- Rx-Anti-Infectives
- Rx-Biological/Blood Rx
- Rx-Cardiology
- Rx-Core Vaccines
- Rx-Corticosteroids
- Rx-Cosmetic
- Rx-Diabetes
- Rx-General Rx
- Rx-Injectable Vitamins
- Rx-Nervous System
- Rx-Ophthalmic
- Rx-Otc And Topicals
- Rx-Rx Services
- Rx-Ulcer Drugs
- Sharps Containers
- Skin & Surgical Prep
- Alcohol/Peroxide Liquid Non-Lab
- Scrub Brushes Impregnanted/Dry
- Scrubs – Liq Surg Prep & Skin Prep Chg
- Scrubs – Liquid Surg Prep & Skin Prep
- Skin Prep Pad/Swab/Appl – Alcohol
- Skin Prep Pad/Swab/Applicators- Chg
- Skin Prep Pad/Swab/Applicators- Other
- Skin Prep Pad/Swab/Applicators- Pvp
- Surgical Prep Wipe-Patient Preop
- Skin Care
- Antifungal Ointments/Sprays: Skin Care
- Bar Soap: Skin Care
- Body Wash, Shampoo, Conditioner
- Body Wash/Shampoo – No Rinse: Skin Care
- Body Wash/Shampoo – Routine: Skin Care
- Cleanse, Body Wash/Shampoo – Routine: Sk
- Cleanse, Lots, Crms, Oints, Balms, Oils
- Cleansers: Wound Care
- Incontinent Ointments/Barriers: Skin Car
- Lots, Crms, Oints, Balms, Oils
- Medicated, Powders/Pastes: Skin Care
- Miscellaneous
- Moisturize, Lots, Crms, Oints, Balms, Oi
- Moisturizers/Lotions/Creams: Skin Care
- Non-Medicated, Powders/Pastes: Skin Care
- Perineal Skin Cleansers: Skin Care
- Powders
- Protect, Skin Prep-Adhsv Rem/Incr Adh
- Shamp/Conditnr/Bdy Wsh/Bth Kt
- Skin Barrier Cleanser, Hand/Face/Body
- Skin Protectants: Skin Care
- Specialty Dressings
- Acetone
- Adhesive Tape Removers: Skin Care
- Aerosol Adhesives
- Alginates: Wound Care
- Anesthetic Creams/Sprays:skin Care
- Antibacterial Ointments/Sprays: Skin Car
- Burn Dressings: Wound Care
- Cleansers: Wound Care
- Collagen: Wound Care
- Composites: Wound Care
- Contact Layers: Wound Care
- Dressings, Hydrogels, Wound Care
- Foams: Wound Care
- Gauze, Wound Care
- Germicidal Dressings
- High Compression System Multi-Layer Wc
- Hydrocolloids: Wound Care
- Impregnated, Wound Care
- Liquid Adhesives
- Liquid Preps, Antimicrobial: Skin Care
- Miscellaneous Wound Care
- Multi-Layer Compression, High Compressio
- Negative Pressure Wound Therapy
- Other Dressings
- Other Ostomy Adhesives
- Other Specialty Dressings, Wet Medicated
- Other, Rx/Otc
- Pastes/Powders/Beads/Granules, Wound Car
- Petroleum Gauze, Wound Care
- Silver/Active Dressings: Wound Care
- Skin Prep, Adhesive Removers
- Skin Substrates: Wound Care
- Transparent Film Dressings: Wound Care
- Transparent Films, Transparent Adhesive
- Wet Medicated Nonadherent Dressings
- Wet Nonmedicated Nonadherent Dressings
- Zinc Paste Bandage (unna Boot) Wound Car
- Sphyg.
- Standard
- Sterile Drapes & Gowns
- Sterile Gauze Sponges
- Sterilization
- Accessories, Container Systems
- Biological Sterile Indicators
- Chemical Sterile Indicators
- Csr Wrap/Ster Pouches/Rolls
- Disposable Sterilizing Wraps
- Glutaraldehyde Sterilant, Sterilization
- Instrument Cleaning Brushes
- Instrument Trays, Sterilization
- Instrument/Equipment Cleaners, Steriliza
- Lubricants, Instrument
- Miscellaneous Sterilization Supplies
- Other Sterilants
- Pouches, Sterilization
- Preclean, Cleaners/Detrgnts/Lubr/Enzymtc
- Preclean/Presoak Sterilants
- Record Systems, Sterilization
- Tapes, Process Indicators Sterilization
- Test Packs, Biological Sterile Indicator
- Test Packs, Chemical Sterile Indicators
- Surface Disinfection
- Cleanroom – Sprays/Wet Wipes/Alcohol
- Cleanroom Dry Wipes
- Floor Mats Absorbent & Dry
- Instrument/Surface Disinf (dnu)
- Solidification Powders
- Spill Kit Bbp/Chemical
- Surface Disinf Alcohol Free Liq/Spray
- Surface Disinf Liq/Spray Alco/Quat/Other
- Surface Disinf Wipes Alcohol/Quat/Other
- Surface Disinf-Dispnsers/Brackets/Stands
- Surface Disinfection Alcohol Free Wipes
- Surface Disinfection Bleach Liq/Spray
- Surface Disinfection Bleach Wipes
- Surface Disinfection Peroxide Liq/Spray
- Surface Disinfection Peroxide Wipes
- Surgical Disposables
- Accessories/Aids, Compression Support
- Basic Surgical Procedure Kp&t-Cus
- Blankets, Forced-Air
- Bone Wires
- Drainage Units, Surgical
- Nonsterile X-Ray Detectable Sponges
- Other Implants & Grafts
- Other Sponges
- Sleeves, Compression Support
- Sterile Laparotomy Sponges
- Sterile X-Ray Detectable Sponges
- Surgical Sponges, Surgical Sponges
- Ventricular Drainage Sets
- Warmers Unit, Blanket
- Wound Drains
- Surgical Equipment/Accessories
- Surgical Instruments
- Bandage Scissors
- Bandage Scissors N/S
- Bandage Scissors Strl
- Biopsy Punches
- Biopsy Punches N/S
- Blade Remover
- Cervical Dilators
- Cervical Dilators N/S
- Conventional Handles
- Conventional Handles N/S
- Conventional Scalpel Blades
- Cpr Emergency Kits
- Dental Hand Instruments
- Dental Hand Instruments N/S
- Dissecting Scissors
- Ear Curets
- Ear Curets N/S
- Ear Piercing Instruments
- Ear Piercing Instruments N/S
- Ear Syringes
- Ear Syringes N/S
- Ent Forceps
- Ent Forceps N/S
- Eye, Scissors: Surgical Instruments
- General Instr, Tools, Laboratory N/S
- General Instruments
- General Instruments N/S
- General Instruments, Tools, Laboratory
- Gravity Enteral Feeding Kits
- Gravity Enteral Feeding Kits N/S
- Implantation Instruments, Orthopedic
- Implantation Instruments, Orthopedic N/S
- Implnts-Screw-Burrs-Saw
- Instrument Sets
- Instrument Sets N/S
- Knives/Blades, Plaster: Orthopedic
- Magnifiers: Surgical Instruments
- Markers, X-Ray Radiological
- Mastoid Retractors
- Mastoid Retractors N/S
- Miscellaneous Endoscopic Supplies
- Miscellaneous Needles & Syringes
- Nasal Specula
- Nasal Specula N/S
- Nasal Specula Strl
- Needle Counters
- Neurological Hammers
- Neurological Hammers N/S
- Operating Room Scissors
- Ophthalmic Occluders
- Other Clamps
- Other Clamps Strl
- Other Surgical Scissors
- Other Surgical Scissors N/S
- Parts, Surgical Instruments
- Percussion Hammers
- Percussion Hammers N/S
- Pessaries
- Pessaries N/S
- Pinwheels: Surgical Instruments
- Pinwheels: Surgical Instruments N/S
- Protectors, Surgical Instruments
- Razors, Skin Prep: Surgical Instruments
- Recapping Devices Syr/Ndl
- Safety Handles N/S
- Sets/Units, Cryosurgery, Surgical Instru
- Skin, Markers: Surgical Instruments
- Specialty Scalpel Blades
- Specimen Forceps
- Splinter Forceps
- Splinter Forceps N/S
- Sponge Counters
- Surgical Access, Other Instrumentation
- Surgical Accessories, Miscellaneous Surg
- Suture Scissors
- Suture Scissors N/S
- Tip Caps, Syringe
- Tip Caps, Syringe N/S
- Tips, Suction
- Tips, Suction N/S
- Tonsil Scissors
- Tuning Forks
- Tuning Forks N/S
- Tweezers: Surgical Instruments
- Tweezers: Surgical Instruments N/S
- Uterine Aspirators N/S
- Utility Forceps
- Utility Forceps N/S
- Table Paper
- Tapes/Wraps
- Applicators, Tubular
- Athletic/Elastic
- Awc
- Bandages
- Bandages, Other Pressure Relief
- Compression Bandages
- Conforming Bandages
- Dressings
- Elastic Bandages
- First Aid Kits
- General Purpose Adhesive Strips
- General Purpose Adhesive Tapes
- Hypoallergenic Adhesive Tapes
- Nonsterile Gauze
- Other Bandages
- Other Tapes/Wraps, Non-Adherent/Burn/Com
- Other Tapes/Wraps, Other
- Other Tapes/Wraps, Other – Bandage/Dress
- Other Tapes/Wraps, Tapes/Band-Aids
- Retainer Bandages
- Self-Adherent Bandages
- Tubular Support Bandages
- Textiles
- Aprons, Staff
- Bath/Hand Towels
- Bedding, Patient, Disposable/Reusable
- Bedspreads
- Bibs, Reusable
- Blanket Reusable
- Dining/Kitchen Linen Reusable
- Drape/Sheets, Nonbedding, Disposable
- Drapes Equipment Covers
- Equipment/Instruments, Covers/Drapes
- Exam Table Headrest Covers
- Infant Scale Liners, Pediatric Supp
- Laundry Bags Reusable
- Mattress Covers Impervious
- Mattress Covers, Textiles
- Other Pillows
- Patient Pillows
- Patient Woven Goods, Other Metal/Plastic
- Patient Woven Goods, Rehabilitation Wear
- Pillow Covers
- Pillows Reusable
- Practice Woven Goods, Other Metal/Plasti
- Professional Disposable Towels
- Rehabilitation Wear
- Robes, Patient
- Sheets/Pillowcases Reusable
- Shroud Kits
- Slippers
- Surgical Equipment Drapes
- Table Covers/Drapes, Surgical
- Textiles, Bedspreads
- Textiles, Blanket Reusable
- Textiles, Pillows Reusable
- Textiles, Textiles
- Towels: Surgical, Disposable
- Towels/Washcloths Reusable
- Warm-Up Wear, Staff
- Therapy Massagers
- Training Equipment & Supplies
- Tympanic/Ear Thermometer Probe Covers
- Uncategorized
- Underpads, Pads & Liners
- Urinalysis Reagents
- Urology
- Access-Irr Tray/Bulb/Syrng
- Catheter Straps/Holders
- Catheters/Foley/Retent/Irrig
- Conventional Hypo Syringes
- Drainage Bags/Tubes
- Facial And Eye Protection
- Irrigtrays/Bulbs/Syringes, Other
- Irrigtrays/Bulbs/Syringes, Tray/Kit, Art
- Male External
- Other Urological Supplies
- Other Urological, Foams
- Straight Intermittent
- Urethral/Foley Trays
- Utensils/Pt Stay/Plas – Disp
- Wound Closure
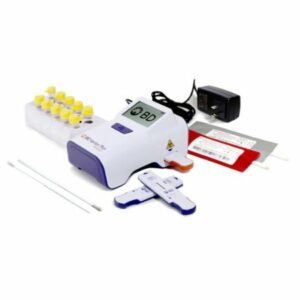
RAPID TEST KIT, SARS-COV-2 & FLU A&B (30TEST)

$809.86
Rapid Test Kit BD Veritor™ System Antigen Detection SARS-CoV-2 / Influenza A + B Direct Anterior Nasal Swab Sample 30 Tests
Out of stock
Item No.
1192774
Categories Flu-Covid Reader Testing, Lab-Respiratory Testing
| Units of Measurement | |
|---|---|
| Manufacturer | BECTON DICKINSON |
| UNSPSC | 41116020 |
| Product Specification Sheet | |
| BD Veritor System COVID-19 and Flu A+B FDA EUA Letter | BD_Veritor_COVID-19_and_Flu_A+B_FDA_EUA_Letter.pdf |
| BD Veritor System COVID-19 and Flu A+B IFU | BD_Veritor_COVID-19_and_Flu_A+B_IFU.pdf |
| BD Veritor System COVID-19 and Flu A+B Patients Fact Sheet | BD_Veritor_COVID-19_and_Flu_A+B_Rapid_Test_Patients_Fact_Sheet.pdf |
| BD Veritor System COVID-19 and Flu A+B Customer Letter April 2021 | BD_Veritor_COVID-19_and_Flu_A+B_Customer_Letter_April_2021.pdf |
| BD Veritor System COVID-19 and Flu A+B Healthcare Providers Fact Sheet | BD_Veritor_COVID-19_and_Flu_A+B_Rapid_Test_Healthcare_Providers_Fact_Sheet.pdf |
| BD Veritor System COVID-19 and Flu A+B Sales Sheet | BD_Veritor_COVID-19_and_Flu_A+B_Sales_Sheet.pdf |
| BD Veritor Systems SARS-CoV-2 Variants July 2021 | BD_Veritor_COVID-19_Variants.pdf |
Related products
-
Covid - Rapids
TEST KIT, SARS ANTIGEN QUICKVUE PROFESSIONAL USE (25/KT)
-
Rsv - Reader Read
CONTROL, VERITOR SYSTEM RSV 10TEST
-
Rsv - Visually Read
TEST KIT, CLEARVIEW RSV (20TEST/KT) WAMPL
-
Rsv - Molecular - Waived
TEST KIT, ALERE I RSV (24/KT)