Introducing the first FDA approved rapid point-of-care test that detects both HIV-1/2 antibodies and free HIV-1 p24 antigen
This 4th generation test has the ability to identify HIV sooner than conventional rapid tests which rely solely on the presence of HIV-1/2 antibodies
Built-in quality controls to let you know the test is working
Can be performed with variety of sample types
Test Strips are provided in an aluminum ziplock pouch and can be separated from each other by tearing along the perforated lines
Each test strip has a cover that is to be removed for sample application and visualization of test results
Chase Buffer included for use with whole blood samples
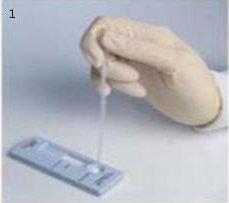
Includes 25 disposable capillary tubes for the collection and transfer of fingerstick samples
Also includes 25 disposable workstations
CLIA Complexity: Moderate For Venous Whole Blood, Serum and Plasma Samples
| Units of Measurement | |
|---|---|
| Manufacturer | ABBOTT RAPID DX |
| UNSPSC | 41116144 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_WAMPL_REAGENT_DETERMINE_HIV_1_2_AG_AB.pdf |
| SDS | SDS_WAMPL_REAGENT_DETERMINE_HIV_1_2_AG_AB.pdf |
| Determine HIV 1-2 Ag-Ab Combo IFU | Determine_HIV-1-2_Ag-Ab_Combo.pdf |
Related Products
Related products
-
Guaiac
DEVELOPER, HEMOCCULT SOL 15ML (20/BX) COULTR
This product has multiple variants. The options may be chosen on the product page -
Guaiac
DEVELOPER, HEMOCCULT-SENSA 15ML BOTTLE COULTR
This product has multiple variants. The options may be chosen on the product page