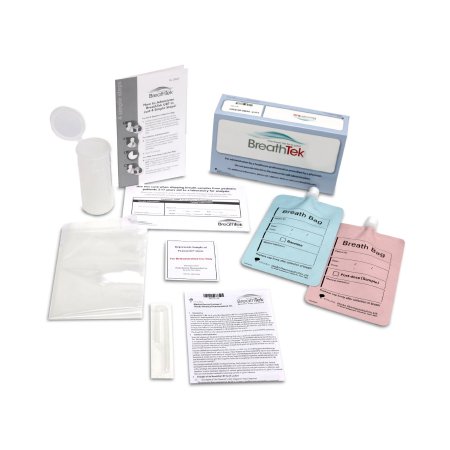
TEST KIT, BREATHTEK UBT F/H. PYLORI (1/KT 5KT/CS)
$381.11
Out of stock
BreathTek® UBT is a noninvasive active testing option for initial diagnosis and post-treatment monitoring of H. pylori infection in patients ages 3 years and older. Active infection determination versus the presence of antibodies, as measured by serology
BreathTek® Urea Breath Test approved to Test and Treat patients in the same office visit or collect samples at multiple sites and courier them to a conveniently located machine. Guidelines recommend a test-and-treat strategy using noninvasive methods, such as UBT, for adults with uninvestigated dyspepsia
Accuracy: 96% Sensitivity and 96% Specificity
During the test, you simply breathe into a small collection bag to capture a base sample of your breath. Then, after drinking a specially formulated drug solution, you breathe into another collection bag. If there is an infection, the bacteria break the solution down into gasses that are measured in your exhaled breath
The process only takes about 20 minutes
Samples are good for 7 days
| Units of Measurement | |
|---|---|
| Manufacturer | OTSUKA AMERICA P |
| UNSPSC | 41116129 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/Breath_Tek_Sales_Aid.pdf |
| Product Spec Sheet | Breath_Tek_Sales_Aid.pdf |
| Otsuka BreathTek UBT H pylori Kit Label Update | Otsuka_BreathTek_UBT_H_pylori_Kit_Label_Update.pdf |
Related Products
Related products
-
Guaiac
TEST KIT, HEMOCCULT II 3SLIDE (102/BX) COULTR
This product has multiple variants. The options may be chosen on the product page -
Ifobt
DEVELOPER, FECAL OCCULT TEST BLOOD SOLUTION ER 10ML (8/BX)
This product has multiple variants. The options may be chosen on the product page