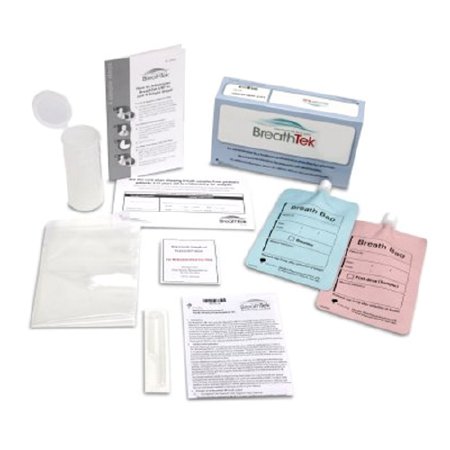
TEST KIT, BREATHTEK UBT F/H. PYLORI (1/KT 5KT/CS)
RESTRICTED - NOT ON FORMULARY
BreathTek® UBT is a noninvasive active testing option for initial diagnosis and post-treatment monitoring of H. pylori infection in patients ages 3 years and older. Active infection determination versus the presence of antibodies, as measured by serology
BreathTek® Urea Breath Test approved to Test and Treat patients in the same office visit or collect samples at multiple sites and courier them to a conveniently located machine. Guidelines recommend a test-and-treat strategy using noninvasive methods, such as UBT, for adults with uninvestigated dyspepsia
Accuracy: 96% Sensitivity and 96% Specificity
| Units of Measurement | |
|---|---|
| Manufacturer | MERIDIAN DIAG IN |
| UNSPSC | 41116129 |
| Product Specification Sheet |
Related Products
Related products
-
Guaiac
DEVELOPER, HEMOCCULT SOL 15ML (20/BX) COULTR
This product has multiple variants. The options may be chosen on the product page -
Ifobt
DEVELOPER, FECAL OCCULT TEST BLOOD SOLUTION ER 10ML (8/BX)
This product has multiple variants. The options may be chosen on the product page