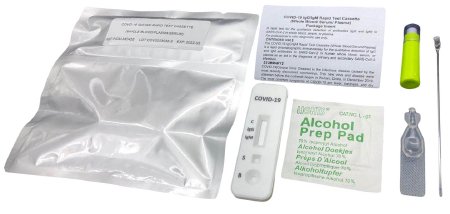
TEST KIT, COVID-19 IGG/IGM WHOLE BLD/SERUM PLASMA (20/CS)
RESTRICTED - NOT ON FORMULARY
Testing of serum, plasma and venous whole blood specimens is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. 263a, that meet requirements to perform moderate or high complexity tests
Testing of fingerstick whole blood specimens is limited to laboratories certified under the Clinical Laboratory Improvement Amendments of 1988 (CLIA), 42 U.S.C. §263a, that meet the requirements to perform high, moderate or waived complexity tests
Product ships from McKesson with minimum 30 days dating
Testing of fingerstick whole blood specimens is authorized for use at the Point of Care (POC), i.e., in patient care settings operating under a CLIA Certificate of Waiver, Certificate of Compliance, or Certificate of Accreditation
RightSign™ COVID-19 IgG/IgM Rapid Test Cassette should not be used to diagnose acute SARS-CoV-2 infection
Laboratories within the United States and its territories are required to report all positive results to the appropriate public health authorities
False positive results may occur due to cross-reactivity from pre-existing antibodies or other possible causes
Due to the risk of false positive results, confirmation of positive results should be considered using second, different IgG or IgM assay
RightSign™ COVID-19 IgG/IgM Rapid Test Cassette is a rapid lateral flow chromatographic immunoassay intended for the qualitative detection and differentiation of IgM and IgG antibodies to SARS-CoV-2 in in human venous whole blood (sodium heparin, EDTA, and sodium citrate), serum or plasma (sodium heparin, potassium EDTA and sodium citrate)
This test uses anti-human IgM antibody (test line IgM), anti-human IgG (test line IgG) and goat anti-mouse IgG (control line C) immobilized on a nitrocellulose strip
| Units of Measurement | |
|---|---|
| Manufacturer | PREMIER BIOTECH |
| UNSPSC | 41116205 |
| Product Specification Sheet | |
| RightSign COVID-19 IgG and IgM Rapid Cassette FDA EUA | CliaWaived_COVID-19_IgG_and_IgM_Rapid_Test_Cassette_FDA_EUA.pdf |
| Premier Biotech COVID-19_IgG-IgM Rapid Test Cassette Package Insert | Premier_Biotech_COVID-19_IgG-IgM_Rapid_Test_Cassette_Package_Insert.pdf |
| RightSign COVID-19 IgG and IgM Rapid Cassette FDA Dec 2020 Authorization | RightSign_COVID-19_IgG-IgM_Rapid_Test_Cassette_FDA_Dec_2020_Letter.pdf |
| RightSign COVID-19 IgG and IgM Rapid Cassette Healthcare Providers Fact Sheet | RightSign_COVID-19_IgG-IgM_Rapid_Test_Cassette_Healthcare_Providers_Fact_Sheet.pdf |
| August 31 FDA EUA200458-S004 Update | FDA_EUA200458-S004_Revision.pdf |
| Rightsign COVID-19 IgG and IgM Rapid Test Cassette Recipients Fact Sheet | RightSign_COVID-19_IgG-IgM_Rapid_Test_Cassette_Recipients_Fact_Sheet.pdf |
| Premier Biotech COVID-19 IgG and IgM Rapid Cassette Quick Ref Format 1 | Premier_Biotech_COVID-19_IgG_and_IgM_Rapid_Cassette_Quick_Reference_Format_1.pdf |
| Premier Biotech COVID-19 IgG and IgM Rapid Cassette Quick Ref Format 2 | Premier_Biotech_COVID-19_IgG_and_IgM_Rapid_Cassette_Quick_Reference_Format_2.pdf |
Related Products
Related products
-
Flu - Reader Read
TEST KIT, INFLUENZA A&B SOFIA (25/KT 12KT/CS)
This product has multiple variants. The options may be chosen on the product page