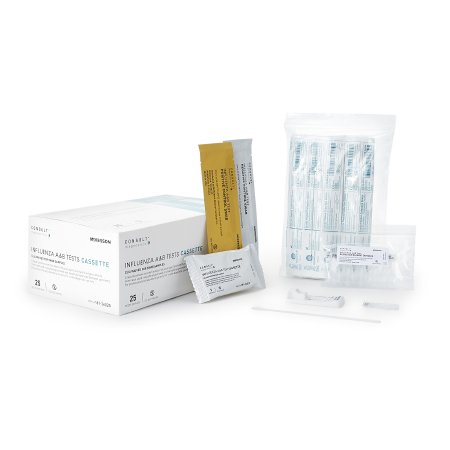
McKesson Consult™ Influenza A & B Tests
Rapid qualitative test that detects Influenza type A and type B antigens directly from nasal swab, nasopharyngeal swab, and Nasopharyngeal aspirate / wash specimens
Color-coded control swab packaging for easy positive/negative identification
Results in under 15 minutes
CONTENTS: 25 Test Cassettes, 25 Sterile Swabs, 25 Extraction Reagent Capsules, 1 Positive Control Swab, 1 Negative Control Swab, 1 Procedure Card, 1 Instructions for Use
CLIA Complexity: Moderate Complexity when used with Nasopharyngeal Wash/Aspirate Samples- CLIA Waived when used with Nasal and Nasopharyngeal Swabs
For aspirate samples, kit sold separately (MFR#: 181-36026)
For professional and laboratory use only
For in vitro diagnostic use only
Not Made with Natural Rubber Latex
- Accessories
- Adaptive Clothing
- Air-Oxygen Masks
- Ancillary Nursing Supplies
- Accessories, Medical Chart
- Applicators, Other Metal/Plastic/Paper S
- Bags, Ice
- Bands/Bracelets, Id Products
- Bath, Sitz
- Bibs, Disposable
- Bins, Storage
- Cotton Rolls
- Cotton/Dacron Swabs
- Cotton/Rayon Balls
- Cover/Lids
- Denture Cups
- Dispensers, Alcohol
- Dressing Jars
- Drinking Cups
- Emesis Bags
- Fire/Rescue Blankets
- Flags Exam Room
- Fracture Bedpans
- Handgrip Covers/Drapes
- Heating Pads, Electric
- Identification Tags
- Labels/Signs
- Leg/Arm Protectors
- Liners – Carafes/Pitchers
- Medical Bags
- Metal Emesis Basins
- Nonmetal Bedpans
- Nonmetal Carafes/Pitchers
- Nonmetal Emesis Basins
- Nonmetal Urinals
- Other Basins
- Other Bowls
- Other Heat/Cold Therapy
- Other Jars
- Other Medical Sundries, Other – Kits/Ute
- Other Medical Sundries, Other Metal/Plas
- Other Medical Sundries, Seals/Ties
- Other Medical Sundries, Utensils/Pt Stay
- Other Swabs
- Other Thermal Products
- Packs, Moist Heat
- Packs/Wraps, Localized
- Patient Belonging
- Personal Belonging Bags
- Proctoscope Swabs
- Racks, Medical Chart
- Solution Bowls
- Sponge Bowls
- Tongue Depressors
- Trays, Food
- Units, Hot Moist Air
- Units, Warming/Cooling, Patient
- Vaginal Swabs
- Wash Basins
- Anesthesia & Suction
- Access-Asprtrs, Comprssr Equip
- Access-Mask,tbng,cnula&mthpc
- Accessories Breathing Circuits
- Accessories, Suction
- Air-Oxygen Masks
- Anesthesia Unit Co2 Absorbants
- Aspirators, Emergency
- Aspirators/Compressors
- Bite Blocks: Surgical Instruments
- Blades, Rigid Laryngoscopes
- Breathing Bags, Respiratory
- Canisters, Collectors, & Canister Sets
- Cannulae, Nasal
- Capnographs Supplies
- Flow Meters & Regulators, Respiratory
- Gas Sampling, Tubing
- Inhalers, Aerosol
- Kits, Custom Suction Canisters & Liners
- Latex Tubing, Med/Surg
- Liners, Suction
- Liquid Oxygen Containers
- Low Volume Aspirators
- Masks/Tubing/Cannulas/Mouthpcs
- Oropharyngeal Airways
- Other Emergency Medical Kits
- Other Impregnated
- Other Tubing, Med/Surg
- Regulators&flowmeters
- Resuscitation, Manual, Respiratory
- Resuscitator Masks
- Suction Canister, Tubing&instr
- Suction, Oral Care
- Tank Cylinders, Carts & Access
- Traps: Respiratory
- Tubing, Suction
- Antibacterials/Anti-Infectives
- Asthma
- Beds And Patient Safety
- Accessories And Parts, Beds
- Accessories, Air Pressure Relief
- Ankle Restraints
- Arm Restraints
- Bed Rails, Beds
- Bed Trays, Beds
- Bedrail Covers
- Beds
- Body Restraints
- Cushions & Positioners
- Electric Beds
- Falls Management Pressure Relief
- Furniture
- Headboards And Footboards, Beds
- Helmets, Protective
- Infant Immobilizers
- Invalid Rings & Cushions
- Lifts And Slings
- Lifts, Lifts/Slings
- Limb Restraints
- Manual Beds
- Mattress Accessories
- Mattress Covers
- Mattress Pads
- Mattresses
- Mattresses/Pads, Stretcher
- Mitts, Restraints
- Non-Powered Pressure Ulcer Mgt (pum)
- Other Cushions
- Other Immobilizers
- Other Patient Restraints & Supports
- Overlays, Pressure Relief (pum)
- Patient Location Alarms
- Patient Room Furniture
- Patient Room Furniture, Furniture
- Patient Room Furniture, Patient Room Fur
- Patient Safety/Fall Management
- Positioning Aids, Patient
- Positioning Aids, Radiographic
- Powered Pressure Ulcer Mgt (pum)
- Retaining Straps, Patient
- Slings, Lifts/Slings
- Supplies, Lifts/Slings
- Vest, Restraints
- Wrist Restraints
- Blood Collection – Containment
- Blood Collection Sets
- Body Care
- Compression
- Containers
- Cpap
- Custom
- Custom & Standard
- Defibrillators
- Diagnostic Cardiology
- Diagnostic Disposables
- Access – Tens Units
- Access-Ekg/Ecg Monitor Electrd
- Accessories, Chargers – Batteries
- Accessories, Ear Irrigation
- Accessories, Oximeters, Respiratory
- Anoscopes
- Batteries – Diag Equipment
- Belts/Straps, Electrode
- Button/Miniature Batteries
- Cables, Ecg Reusable
- Calipers, Ecg
- Covers, Ultrasonic Probe
- Diagnostic Tools, Phys Med/Rehab
- Disposable, Cuffs, Sphyg.
- Distilled/Deionized Water
- Ear Probe Tips
- Ear Specula
- Ecg Recording Paper
- Electrocardiographic Electrodes
- Electrocardiographic Neonatal Electrodes
- Electrode Coupling Gel
- Envelopes Storage, X-Ray Film
- Fetal Heart Monitoring Paper, Ultrasonic
- Filters, Sterilization
- Food
- Gel Warming Units, Electromedical
- Instrument Batteries
- Kits, Ear Irrigation
- Leads Ecg Reusable
- Light Bulbs, Endoscopic
- Lighting Bulbs, Fixtures, Parts
- Lubricating Disposables
- Misc Diag Supplies/Acces
- Mouthpieces/Nose Clips, Respiratory
- Office Supplies
- Oral/Rectal/Aux Elec Therm Probe Covers
- Other Batteries
- Other Disposables, Medicine Cups
- Other Electrode Accessories
- Other Enema Products
- Other Radiological
- Other Recording Paper
- Other Ultrasonic Detectors
- Otoscope Disposables, Light Bulbs, Endos
- Pouches, Telemetry, Electromedical
- Printer Paper
- Pulmonary Spiro Access
- Sheet X-Ray Film
- Specula, Post Surgical Garments
- Tape Measure
- Temporal/Forehead – Contact Probe Covers
- Throat, X-Ray Shield
- Tympanic/Ear Thermometer Probe Covers
- Ultrasonic Coupling Gel
- Vaginal Specula
- View Boxes, X-Ray
- Diagnostic Imaging
- Disposable Underwear
- Durable Medical Equipment
- Adl, Medication Organizers/Dispensers Ad
- Aluminum Canes
- Animal Skin, Pressure Relief
- Bath Mats
- Bathing Adl
- Cane Parts
- Canes
- Commode Chairs
- Covers, Urinal
- Eating And Drinking Adl
- Grooming And Dressing Adl
- Lumbar Cushions
- Other Adl Products
- Other Canes
- Pails, Commode
- Protectors, Heel/Elbow
- Rails/Grab Bars, Ambulatory Aids
- Reaching And Gripping Adl
- Rollators
- Shower/Bath Chairs
- Toilet Aids Adl
- Walker Parts
- Walkers
- Wheelchair Cushions
- Wheelchair Parts
- Wheelchairs
- Wooden Canes
- Electronic Wearables
- Exam & Patient Room Furnishing
- Baby Seats & Strollers
- Bins, Shelving/Parts
- Blood Drawing Chairs
- Cabinets/Wall Desks
- Carts
- Communication Aids/Equipment
- Cubicle Curtains, Reusable
- Enuresis Alarms
- Headlights: Surgical Instruments
- Heel Stirrup Covers
- Infant Blankets
- Instrument/Equipment Carts
- Kick Buckets
- Lift Chairs
- Lighting/Heat Lamps
- Lights, Examination
- Lights, Surgical
- Mayo Stands & Trays
- Other Alarm Products
- Other Cabinets
- Other Carts
- Other Chairs
- Other Equipment, Furniture
- Other Fixtures, Other – Furniture/Equipm
- Parts, Carts
- Penlights
- Printers
- Recliner Chairs
- Screens, Privacy
- Service/Utility Carts
- Stools
- Stretchers
- Stretchers, Stretchers Transfer Backboar
- Stump Socks
- Surgical Room Furn & Equip
- Exam Tables
- Feeding Supplies
- Adapters And Connectors
- Feeding Pump Accessories
- Feeding Pump Backpacks
- Feeding Pump Batteries
- Feeding Pump Repair Parts
- Gastrostomy Tubes
- Gastrostomy Tubes – Full Length
- Gravity Bags
- Jejunal Tubes – Full Length
- Nasogastric Tubes
- Oral Tip Syringes
- Other Enteral Feeding Accessories
- Reusable Irrigating Syringes
- Securement Devices
- Spike Sets
- Tube Decloggers
- Tube Holders
- Fluff Dressings
- Food Service Disposables
- Food Supplements
- Diabetic: Enteral Tube Feeding
- Digestive Aids/Laxatives
- Elemental And Semi-Elemental
- Enteral Tube Feeding
- Enteral Tube Feeding, Nutritional-Condit
- Feed Supplies-Infant Nursing
- Food/Beverage Thickeners
- Immune Enhancing: Enteral Tube Feeding
- Nutrient Dense Supplements: Medical Nutr
- Nutritional-Condition Specific
- Nutritional-Modular Components
- Nutritional-Modular Components, Critical
- Nutritionals – Infant
- Nutritionals – Metabolics
- Nutritionals – Miscellaneous
- Nutritionals – Pediatric
- Nutritionals-Oral Supplements
- Oral Nutritional Supplements: Medical
- Oral Supplements – Diabetic
- Oral Supplements – Wound Care
- Other Enteral Tube Feeding Formulae
- Other Medical Nutritional/Supplements
- Other Supplements, Nutritionals – Miscel
- Pediatric/Infant Formula: Medical Nutrit
- Pediatrics, Nutritionals – Infant
- Pulmonary: Enteral Tube Feeding
- Renal: Enteral Tube Feeding
- Standard: Enteral Tube Feeding
- Total Pediatric: Enteral Tube Feeding
- Vitamins/Supplements
- Wound Healing: Enteral Tube Feeding
- Gauze
- Combine Dressings
- Dressings, Germicidal Dressings
- Dry Nonadherent Dressings
- Eye Patches
- Fluff Dressings
- Nasal Trusses
- Nonsterile Gauze Sponges
- Nonsterile Gauze, Roller Gauze Bandages
- Nonsterile Nonwoven Sponges
- Packers Gauze Bandages
- Sterile Gauze , Roller Gauze Bandages
- Sterile Gauze Sponges
- Sterile Nonwoven Sponges
- Gloves
- Autopsy/Utility/Housekeeping
- Cleanroom Gloves – Other
- Cut Resistant Gloves
- Dispensers, Glove
- Glove Box Holders/Dispensers
- Glove, Exam Latex Nonstr Pf
- Glove, Exam Latex Nonstr Pf W/Emoll
- Glove, Exam Latex Sterile Pf
- Glove, Exam Latexfree Nonstr Pf
- Glove, Exam Nitrile Nonstr Pf
- Glove, Exam Nitrile Nonstr Pf W/Emoll
- Glove, Exam Nitrile Sterile Pf
- Glove, Exam Vinyl Nonstr Pf
- Glove, Exam Vinyl Sterile Pf
- Glove, Surg Latex Pf
- Glove, Surg Latexfree Pf
- Glove, Surg Latexfree Pf W/Emoll
- Gloves Nitrile Clean Room – Sterile
- Other Gloves
- Other Gloves, Finger Cots
- Pressure Gloves
- Utility Gloves
- Hand Hygiene
- Hand Hygiene, Wipes, Personal Care: Skin
- Hand Sani – Alcoh Fr Desp Refill Btl/Bag
- Hand Sani – Alcohol Desp Refill Btl/Bag
- Hand Sani/Soaps- Disp/Brackets/Stands
- Hand Sanitizer – Alcohol Btl/Bag
- Hand Sanitizer – Alcohol Free Btl/Bag
- Hand Sanitizer – Wipes
- Hand Soap/Scrb – Foam Des Refill Btl/Bag
- Hand Soap/Scrb – Liq Desp Btl/Bag Other
- Hand Soap/Scrb – Liq Desp Refill Btl/Bag
- Hand Soaps/Scrubs – Foam Btl/Bag
- Hand Soaps/Scrubs – Waterless No Rinse
- Ip Hygiene Stations And Other
- Other Skin Care Products(dnu)
- Hand Hygiene/Surface Disinfect
- Alcohol
- Alcohol Based Hand Sanitizer
- Alcohol Preps
- Alcohol-Free Hand Sanitizer
- All Other Infection Prevention
- Antiseptic Preps: Skin Care
- Bleaches: Housekeeping
- Chemical Spill Kits
- Cleanroom – Alcohol
- Cleanroom – Sprays/Wet Wipes
- Cleanroom Dry Wipers
- Floor Mats
- Hand Hygiene, Disposable Wash Cloths
- Hand Hygiene, Wipes, Personal Care: Skin
- Hand Sanitizer Dispenser Equipment
- Hand Sanitizer Wipes
- Infectious/Cleanup/Turnover Spill Kits
- Instrument/Surface Disinfectants
- Iodophor Preps: Skin Care
- Iodophors: Skin Care
- Laundry Bags,infection Control
- Liquid Scrubs, Antimicrobial: Skin Care
- Liquid Soaps: Skin Care
- Liquid/Foam Hand Soaps-No Rinse:skin Car
- Other Hazardous Waste Control Products
- Other Housekeeping
- Other Skin Care Products
- Other Spill Kits
- Peroxides
- Reclosable Bags
- Red Bag Infectious Waste Control
- Scrub Sponges, Antimicrobial: Skin Care
- Skin & Surgical Prep, Wipes, Personal Ca
- Skin Prep
- Solidification Treatment, Spill Kits
- Spill Kits
- Surface Disinfectation Wipes
- Surface Disinfection
- Waste Receptacles, Contaminated
- Wipes
- Home Environments
- Housekeeping
- Bags Kick Bucket
- Bags Laundry
- Bags Laundry Biohazard/Ylw
- Bags Laundry Watersoluble
- Bags Misc/Accessories
- Bags Other Chemo Transport
- Bags Paper
- Bags Trash 12-16 Gal
- Bags Trash 20-30 Gal
- Bags Trash 31-35 Gal
- Bags Trash 4-11 Gal
- Bags Trash 40-50 Gal
- Bags Trash 55-65 Gal
- Bags Zipper
- Bags Zipper Amber
- Bio Hazard – Other
- Chemical Bath/Toilet/Urinal
- Chemical Carpet/Stain Remover
- Chemical Cleaners
- Chemical Degreasers
- Chemical Dishwashing
- Chemical Dusting/Polishing
- Chemical Floor Cleaning
- Chemical Floor Finishing
- Chemical Floor Stripping
- Chemical General Cleaning
- Chemical Glass
- Chemical Laundry
- Chemical Misc
- Chemical Odor Control
- Clean Rm Accessories Papr/Pads/Tacky Mat
- Facial Tissue & Dispensing
- Hardware Bottle/Trigger Empty
- Hardware Brooms/Dust Pans
- Hardware Buckets/Accessories
- Hardware Cart Covers
- Hardware Carts
- Hardware Cloth/Sponge/Scrubber
- Hardware Floor Machines/Parts
- Hardware Hampers/Accessories
- Hardware Misc
- Hardware Mops/Squeegees
- Hardware Mops/Squeegees/Mopheads
- Hardware Storage Bin/Organizer
- Hardware Trash Can/Accessories
- Hardware Vacuum/Sweeper/Parts
- Janitorial Supplies
- Kitchen Supplies
- Napkins & Dispensing
- Other Bags
- Paper Prod (towels/Tisues/Tp)
- Reclosable Bags
- Red Bag Inf Waste- Autoclavable
- Red Bag Infectious Waste Control
- Seat Covers & Dispensing
- Toilet Tissue & Dispensing
- Towels & Tissue, Towels Roll & Dispensin
- Towels C/M/S Fold & Dispensing
- Towels C/M/S Fold And Dispensi
- Towels Roll & Dispensing
- Trash Can Liners
- Trash Receptacles/Hampers
- Wipes Dry & Dispensing
- Yellow Bag Infectious Waste Control
- Zipper Lock/Dietary Bags
- Inco Wipes
- Incontinence Briefs
- Interpretive Ecg
- IV Therapy
- Adapters/Connectors, Miscellaneous Urolo
- Adapters/Connectors, Vials, Medicine
- Admin Sets
- Admin Sets, Blood Collection Needle Sets
- Administration Sets, Parenteral
- Ambulatory Infusion Sets, Parenteral
- Arm Board Covers
- Arm Boards & Accessories
- Connector Cap Disinfection, Parenteral
- Connectors & Adapters, Parenteral
- Connectors Needless, Parenteral
- Cstd
- D5w-500ml
- Dispenser IV Fluid, Parenteral
- Immobilizers, IV Catheter/Needle
- Infusion Pump Accessories & Other
- Infusion Pump Asset Return Boxes
- Infusion Pump Batteries
- Infusion Pump Medication Bags
- Infusion Pump Pouches
- Infusion Pump Repair Parts
- Infusion Pump Sets
- Infusion Pump Sets – Lvp
- Intraosseous Infusion Needles
- Intravenous Poles
- IV Fluid Containers, Parenteral
- IV Pumps, Infusion Pumps & Equipment
- Lactated Ringer-500ml
- Medicine Kp&t-Cus
- Multi-Use, Extension Sets
- Other – IV Solutions
- Other Cardiac Catheter Accessories
- Other Emergency Medical Equip/Supplies
- Other IV Therapy, Kits
- Other IV Therapy, N/S Accessories
- Other Parenteral Supplies
- Other Renal Dialysis Supplies
- Other, Other IV Therapy
- Pump Accessories
- Safety IV Catheters
- Security Seals
- Sod. Chl. – Irr
- Standard Huber Needles/Sets
- Standard IV Catheters
- Sterile Water – Irr
- Tourniquet Straps
- Tpn Bags And Sets
- Transfer Device, Transfer/Access Devices
- Winged IV Catheters,safety,parenteral
- Kit/Tray Sml Wound/Lac/Incis/D
- Kit/Tray Sml Wound/Lac/Incis/Dsg
- Kits
- Kits, Custom & Standard
- Cleanup/Turnover Kits Standard
- Custom, Kit Specialty/ Personal/Admiss
- Custom, Kit/Tray Sml Wound/Lac/Incis/Dsg
- Custom, Tray/Kit Anesthesia/Pain
- Custom, Tray/Kit General/Endo/Gi
- Custom, Tray/Kit Ob Gyn Gu
- Custom, Tray/Kit Proc Plastics
- Other Standard Kits & Trays
- Standard, Kit Specialty/ Personal/Admiss
- Standard, Kit/Tray Sml Wound/Lac/Incis/D
- Standard, Tray/Kit General/Endo/Gi
- Standard, Tray/Kit Ob Gyn Gu
- Standard, Tray/Kit, Proc Eent/Ophthalmic
- Standard, Tray/Kit, Proc Ortho/Neuro
- Lab – Cardiac Marker
- Lab – Poc Chemistry
- Lab-Blood Bank
- Lab-Blood Glucose Meters & Sup
- Lab-Chemistry
- Lab-Coagulation
- Lab-General Lab Equipment
- Lab-Hematology
- Lab-Immunoassay
- Lab-Instrument Driven Testing
- A1c Accessories
- A1c Analyzers
- A1c Cals/Ctrls
- A1c Reagents
- Cardiac Marker Reagents
- Hemoglobin Reagents
- Lipid Accessories
- Lipid Reagents
- Pt/Inr Accessories
- Pt/Inr Analyzers
- Pt/Inr Cals/Ctrls
- Pt/Inr Reagents
- Quantitative Glucose Analyzers
- Quantitative Glucose Reagents
- Urinalysis Accessories
- Urinalysis Analyzers
- Urinalysis Analyzers Promo Bundle
- Urinalysis Reagents
- Waived Chemistry Accessories
- Waived Chemistry Reagents
- Waived Hematology Consumables
- Waived Hematology Reagents
- Wbc Reagents
- Lab-Lab Services
- Lab-Lab Supplies
- Lab-Microbiology
- Lab-Molecular
- Lab-Pathology
- Lab-Rapids
- Lab-Respiratory Testing
- Lab-Specimen Collection
- Lab-Urinalysis
- Miscellaneous Respiratory
- Needles & Syringes
- Conventional Hypo Combos
- Conventional Hypo Needles
- Conventional N&s, Conventional Hypo Syri
- Conventional Pen Needles
- Conventional Tb Needle/Syr/Combo
- Dnu / Delete Post Movement Of Items
- Misc / Other, N/S Accessories
- Misc / Other, Pressure Monitoring/Stopco
- N/S Accessories
- Other Syringes – Legend
- Safety Hypo Needle Hinged/Flip
- Safety Hypo Needle/Syr Combo Hinged/Flip
- Safety Hypo Needle/Syr Combo Retractable
- Safety Hypo Needle/Syr Combo Sliding
- Safety Insulin Pen Needle
- Safety Needle Combos – Hinged Pivoting
- Safety Needle Combos – Shielding
- Safety Needle Combos – Sliding Sleeve
- Safety Needles – Hinged/Pivoting
- Safety Syr/Needles – Retracting &combos
- Safety Tb Needle/Syr/Combo
- Specialty Conventional – Tb, Insulin
- Spinal Needles (quincke, Tuohy)
- Syringe 20cc/25cc/30cc/35cc
- Syringe 3cc/5cc/6cc/7cc
- Syringe Bulb/Irrigation/Cath Tip
- Syringe Oral/Enteral/Enfit
- Office Supplies
- All Other Office Supplies, General Offic
- All Other Office Supplies, Industrial
- All Other Office Supplies, Other
- All Other Office Supplies, Technology
- Binders & Chart Holders
- Coloring Books, Stickers, Toys
- Essendant Office Supplies, General Offic
- Essendant Office Supplies, Industrial
- Food Service
- Medical & Patient Forms
- Other Office Supplies
- Standard Batteries
- Orthopedic
- Orthopedics
- Abdominal Binders
- Accessories, Paraffin Baths
- Aluminum Crutches
- Arch Orthoses/Supports
- Arm Slings
- Arm Slings/Elevators/Elbow
- Arm Splints/Supports
- Back, Orthoses/Splints/Supports
- Balance, Exercise
- Balls, Exercise Phys Med/Rehab
- Bands/Putty, Exercise
- Blankets, Circulating Fluid
- Boots, Cast Products
- Cast Protectors, Orthopedic
- Cast Shoes
- Cast Shoes/Post Op Shoes
- Casting Prod(tape/Plast/Pad)
- Cervical Collars Acute Care
- Cervical Collars Extrication
- Cervical Collars Foam
- Cervical Collars Other
- Cervical Collars Rehabilitation
- Cervical Cushions
- Chest Binders
- Clavicle Straps
- Cpm Lower Limb Exercise
- Crutch Parts
- Crutches
- Elbow Immobilizers
- Elbow, Splints/Supports
- Electric Cast Cutters
- Electroanalgesic Units, Tens
- Electroanalgesic, Tens Electrodes
- Finger Splints
- Foot/Ankle, Orthoses/Splints/Supports
- Hand, Splints/Supports
- Hba Products
- Heel Liners
- Heel Stirrups: Orthopedic
- Hernia Trusses
- Hip, Orthoses/Supports
- Hot Water Bags/Bottles
- Insole Liners
- Knee Immobilizers
- Knee, Orthoses/Splints/Supports
- Leg, Orthoses/Splints/Supports
- Lower, Extremity Exercisers
- Lumbosacral Supports
- Massagers, Physical Medicine/Rehab
- Mats, Exercisers
- Moleskins, Pressure Relief
- Neuromuscular Stimulator Electrodes
- Orthopedic Shoes
- Orthotic/Shoe Inserts
- Other Cast Products: Orthopedic
- Other Crutches
- Other Orthopedics Supplies, Contoured Su
- Other Orthopedics Supplies, Finger Cots
- Other Orthopedics Supplies, General Immo
- Other Orthoses/Splints/Supports
- Other Physical Medicine/Rehabilitation
- Other Physical Therapy
- Other Soft Goods
- Other Traction Supplies
- Other, Other Orthopedics Supplies
- Padding, Cast Products
- Pads, Circulating Fluid
- Parallel Bars: Phys Med/Rehab
- Physical Therapy, Physical Therapy
- Plaster, Cast Products
- Pneumatic Splints/Supports
- Protectors, Finger
- Pumps, Circulating Fluid
- Shoulder Immobilizers
- Shoulder, Orthoses/Splints/Supports
- Sleeves/Protectors, Pressure Relief
- Soft Goods, Other Pressure Relief
- Soft Goods, Overlays, Pressure Relief (p
- Soft Goods, Rib Belts/Abdominal/Back Sup
- Soft Goods, Wrist/Forearm/Finger Splints
- Spine Boards
- Splinting Devices: Orthopedic
- Stairs, Exercisers
- Stockinettes
- Supplies, Exercisers
- Suspensories/Trusses
- Synthetic, Cast Products
- Systems, Traction
- Tens Cables/Leads
- Toe, Splints/Supports
- Transfer Boards, Beds
- Trapeze Exercisers
- Ultrasound Units: Phys Med/Rehab
- Units, Paraffin Baths
- Upper, Extremity Exercisers
- Walking Heels: Orthopedic
- Water Cushion, Pressure Relief
- Weights, Exercisers
- Wooden Crutches
- Wrist Immobilizers
- Wrist,orthoses/Splints/Supports
- Ostomy
- Adult Bags W/O Anti-Reflux Valve Urinary
- Appliance Belts, Ostomy
- Bile Collection Bags
- Collection Bags, Ostomy
- Fecal Units
- Ileostomy Appliance Kits
- Inserts, Ostomy
- Irrigators, Ostomy
- Mini Ostomy Pouches
- Odor Control Products, Ostomy
- One Piece Ostomy System, Closed
- One Piece Ostomy System, Drainable
- One Piece, Urostomy
- Ostomy Supplies
- Other, Urostomy
- Pastes & Powders: Ostomy
- Skin Barriers, Wafers: Ostomy
- Sleeves, Ostomy Irrigators
- Stoma Caps, Ostomy
- Tail Closures, Ostomy
- Two Piece Ostomy System, Closed
- Two Piece Ostomy System, Drainable
- Two Piece, Urostomy
- Wafers W/Flange: Ostomy
- Other Sizes
- Patient Assessment/Monitoring
- Accessories, Sphyg.
- Audiometers
- Audiomtry/Otoscope/Ophthalmic
- Baby Analog Scales
- Baby Digital Scales
- Balances, Electronic
- Battery Operated Endoscopic Power
- Calibrators, Spirometer/Pneumotachometer
- Chair Scales
- Computer Parts
- Diagnostic Spirometers
- Disposable Forehead Thermometer Strips
- Electronic Stethoscopes
- Electronic, Units, Sphyg.
- Eye, Color Discrimination Charts
- Eye, Visual Acuity Charts
- Floor Analog Scales
- Floor Digital Scales
- Glass Patient Thermometers
- Handles, Rigid Laryngoscopes
- Inflation Sets, Sphyg.
- Line Operated Endoscopic Power
- Manual Blood Pressure
- Manual, Units, Sphyg.
- Miscellaneous Electromedical Supplies
- Monitors, Auto Bp
- Ophthalmo/Otoscope Sets
- Ophthalmoscopes
- Oral/Rectal/Aux Dig Stick Thermometers
- Oral/Rectal/Aux Disposable Thermometers
- Oral/Rectal/Aux Elec Therm With Probe
- Other – Diag Supplies/Access
- Other Auditory
- Other Cables & Leads
- Other Ophthalmic
- Other, Scales
- Otoscopes
- Parts/Accessories Scales
- Physiologic Monitoring Systems
- Probes, Oximeter, Respiratory
- Pulse Oximeter, Respiratory
- Retinoscopes
- Reusable, Cuffs, Sphyg.
- Scales / Height Rods, Tape Measure
- Specialty Patient Thermometers
- Standard Stethoscopes
- Stethoscope Parts
- Temporal Thermometer (contact)
- Temporal Thermometer (non-Contact)
- Thermometer Replacement Parts
- Tonometers: Ophthalmological
- Tympanic/Ear Thermometers
- Visual Function Analyzers
- Wheelchair Scales
- Personal Care
- Body Care, Personal Care – Patient
- Body Care, Shamp/Conditnr/Bdy Wsh/Bth Kt
- Body Care, Wipes Dry & Dispensing
- Bodywash_soap
- Bodywash_soap No Rinse
- Breast Pumps & Accessories
- Condoms
- Dental Supplies
- Denture Adhesive
- Denture Cleaner
- Deodorants_antiperspirants
- Face_lips_ears
- Feminine Hygiene
- Flossing
- Freshener
- Hair Care
- Hair Combs_brushes
- Hair Shampoo_conditioner
- Hair Shampoo_conditioner No Rinse
- Maternity
- Mouthwash
- Nail Care
- Nail Clippers
- Oral Care, Personal Care – Patient
- Personal Care-Feminine Hygiene
- Prsnl Care-Nursng Pd/Brst Pump
- Sanitary/Ob Pads
- Shaving Cream
- Shaving Razors
- Shaving Skin Conditioner
- Shaving, Shaving Skin Conditioner
- Suction
- Swab_toothette
- Tampons
- Toothbrushes
- Toothpaste
- Personal Care – Patient
- Personal Protective Equipment
- Accessories, Respirator Masks
- Cleanroom Frocks, Sleeves And Other
- Cleanroom Gown Sterile
- Cleanroom Headwear/Hoods
- Cleanroom Shoecovers/Boots
- Coveralls, Staff
- Coveralls, Staff – Cleanroom
- Ear Plugs
- Eyeglasses
- Face Shields & Accessories
- Gowns Chemo
- Head Coverings, Surgical
- Isolation Gowns, Reusable, Staff
- Isolation/Procedure Gowns (aami 2 Level)
- Isolation/Procedure Gowns (aami 3 Level)
- Isolation/Procedure Gowns (aami 4 Level)
- Isolation/Procedure Gowns (aami I Level)
- Isolation/Procedure Gowns (fluid Resist)
- Isolation/Procedure Gowns (not Rated)
- Lab Coats, Disposable,staff
- Lab Coats, Reusable, Staff
- N95 Mask Accessories
- N95 Medical Masks
- N95 Or Greater Filter Industrial Mask
- Other – Apparel/Personal Prot
- Other Safety Products
- Papr & Accessories
- Patient Gowns Pediatric Disposable
- Patient/Consumer Protective Face Masks
- Patient/Exam Gowns Disposable
- Patient/Exam Gowns Reusable
- Pediatric Patient Gowns Reusable
- Procedure Mask (astm Level 1)
- Procedure Mask (astm Level 2)
- Procedure Mask (astm Level 3)
- Procedure Mask (not Rated)
- Respirator Mask, Gas Masks & Accessories
- Safety Glasses & Accessories
- Safety Goggles
- Scrub Suits, Disposable
- Scrub Suits, Reusable
- Shoe Covers, Surgical
- Staff Disposable
- Staff Ears, Ear Plugs
- Staff Eyes, Facial And Eye Protection
- Surgical Mask (astm Level 1)
- Surgical Mask (astm Level 2)
- Surgical Mask (astm Level 3)
- Surgical Mask (not Rated)
- Pharmaceutical Feed
- Pharmaceutical Product
- Procedure Equipment (sterilize
- Respiratory Therapy
- Accessories/Parts Allied Healthcare Prod
- Adapters & Connectors, Respiratory
- Aerosol / Nebulizer Masks
- Aerosol / Nebulizer Tubing
- Bronchial/Exerciser Spirometers
- Brushes, Tracheal Tubes
- Buttons, Tracheostomy (plugs)
- Connector
- Filters, Respiratory
- Heat/Moisture Exchange
- Holders, Endotracheal Tubes
- Holders, Tracheostomy Tubes
- Humidifiers
- Inner Cannula, Tracheostomy
- Misc Humidification
- Misc Trach
- Miscellaneous Respiratory
- Mouth-To-Mask Cpr Resuscitation Masks
- Nasal Aspirators
- Nebulizer Access: Mouthpiece/Tee/Tubes
- Nebulizer Compressor (equipment)
- Nebulizer Kits, Handheld (no Equipment)
- Nebulizer Parts: Repair/Replac/Filters
- Other Respiratory, Adapter/Connectors
- Oxyg Concentrator/Compressor Accessories
- Oxyg Concentrator/Compressor Units
- Oxygen Concentrator Filters
- Oxygen Connecting Tubing
- Straps, Tracheal (twill Tape)
- Stylets, Tracheal
- Suction Catheters Trays Closed System
- Suction Catheters Trays Open System
- Suction Catheters With Valve (single)
- Surgical Aspirators
- Trach Cleaning Kits
- Valves, Tracheostomy (speaking)
- Vent Accessories & Other
- Vent Filters
- Vent Repair Parts
- Rig Components
- Rigs
- Rx – Asthma
- Rx-Anti-Infectives
- Rx-Biological/Blood Rx
- Rx-Cardiology
- Rx-Core Vaccines
- Rx-Corticosteroids
- Rx-Cosmetic
- Rx-Diabetes
- Rx-General Rx
- Rx-Injectable Vitamins
- Rx-Nervous System
- Rx-Ophthalmic
- Rx-Otc And Topicals
- Rx-Rx Services
- Rx-Ulcer Drugs
- Sharps Containers
- Skin & Surgical Prep
- Alcohol/Peroxide Liquid Non-Lab
- Scrub Brushes Impregnanted/Dry
- Scrubs – Liq Surg Prep & Skin Prep Chg
- Scrubs – Liquid Surg Prep & Skin Prep
- Skin Prep Pad/Swab/Appl – Alcohol
- Skin Prep Pad/Swab/Applicators- Chg
- Skin Prep Pad/Swab/Applicators- Other
- Skin Prep Pad/Swab/Applicators- Pvp
- Surgical Prep Wipe-Patient Preop
- Skin Care
- Antifungal Ointments/Sprays: Skin Care
- Bar Soap: Skin Care
- Body Wash, Shampoo, Conditioner
- Body Wash/Shampoo – No Rinse: Skin Care
- Body Wash/Shampoo – Routine: Skin Care
- Cleanse, Body Wash/Shampoo – Routine: Sk
- Cleanse, Lots, Crms, Oints, Balms, Oils
- Cleansers: Wound Care
- Incontinent Ointments/Barriers: Skin Car
- Lots, Crms, Oints, Balms, Oils
- Medicated, Powders/Pastes: Skin Care
- Miscellaneous
- Moisturize, Lots, Crms, Oints, Balms, Oi
- Moisturizers/Lotions/Creams: Skin Care
- Non-Medicated, Powders/Pastes: Skin Care
- Perineal Skin Cleansers: Skin Care
- Powders
- Protect, Skin Prep-Adhsv Rem/Incr Adh
- Shamp/Conditnr/Bdy Wsh/Bth Kt
- Skin Barrier Cleanser, Hand/Face/Body
- Skin Protectants: Skin Care
- Specialty Dressings
- Acetone
- Adhesive Tape Removers: Skin Care
- Aerosol Adhesives
- Alginates: Wound Care
- Anesthetic Creams/Sprays:skin Care
- Antibacterial Ointments/Sprays: Skin Car
- Burn Dressings: Wound Care
- Cleansers: Wound Care
- Collagen: Wound Care
- Composites: Wound Care
- Contact Layers: Wound Care
- Dressings, Hydrogels, Wound Care
- Foams: Wound Care
- Gauze, Wound Care
- Germicidal Dressings
- High Compression System Multi-Layer Wc
- Hydrocolloids: Wound Care
- Impregnated, Wound Care
- Liquid Adhesives
- Liquid Preps, Antimicrobial: Skin Care
- Miscellaneous Wound Care
- Multi-Layer Compression, High Compressio
- Negative Pressure Wound Therapy
- Other Dressings
- Other Ostomy Adhesives
- Other Specialty Dressings, Wet Medicated
- Other, Rx/Otc
- Pastes/Powders/Beads/Granules, Wound Car
- Petroleum Gauze, Wound Care
- Silver/Active Dressings: Wound Care
- Skin Prep, Adhesive Removers
- Skin Substrates: Wound Care
- Transparent Film Dressings: Wound Care
- Transparent Films, Transparent Adhesive
- Wet Medicated Nonadherent Dressings
- Wet Nonmedicated Nonadherent Dressings
- Zinc Paste Bandage (unna Boot) Wound Car
- Sphyg.
- Standard
- Sterile Drapes & Gowns
- Sterile Gauze Sponges
- Sterilization
- Accessories, Container Systems
- Biological Sterile Indicators
- Chemical Sterile Indicators
- Csr Wrap/Ster Pouches/Rolls
- Disposable Sterilizing Wraps
- Glutaraldehyde Sterilant, Sterilization
- Instrument Cleaning Brushes
- Instrument Trays, Sterilization
- Instrument/Equipment Cleaners, Steriliza
- Lubricants, Instrument
- Miscellaneous Sterilization Supplies
- Other Sterilants
- Pouches, Sterilization
- Preclean, Cleaners/Detrgnts/Lubr/Enzymtc
- Preclean/Presoak Sterilants
- Record Systems, Sterilization
- Tapes, Process Indicators Sterilization
- Test Packs, Biological Sterile Indicator
- Test Packs, Chemical Sterile Indicators
- Surface Disinfection
- Cleanroom – Sprays/Wet Wipes/Alcohol
- Cleanroom Dry Wipes
- Floor Mats Absorbent & Dry
- Instrument/Surface Disinf (dnu)
- Solidification Powders
- Spill Kit Bbp/Chemical
- Surface Disinf Alcohol Free Liq/Spray
- Surface Disinf Liq/Spray Alco/Quat/Other
- Surface Disinf Wipes Alcohol/Quat/Other
- Surface Disinf-Dispnsers/Brackets/Stands
- Surface Disinfection Alcohol Free Wipes
- Surface Disinfection Bleach Liq/Spray
- Surface Disinfection Bleach Wipes
- Surface Disinfection Peroxide Liq/Spray
- Surface Disinfection Peroxide Wipes
- Surgical Disposables
- Accessories/Aids, Compression Support
- Basic Surgical Procedure Kp&t-Cus
- Blankets, Forced-Air
- Bone Wires
- Drainage Units, Surgical
- Nonsterile X-Ray Detectable Sponges
- Other Implants & Grafts
- Other Sponges
- Sleeves, Compression Support
- Sterile Laparotomy Sponges
- Sterile X-Ray Detectable Sponges
- Surgical Sponges, Surgical Sponges
- Ventricular Drainage Sets
- Warmers Unit, Blanket
- Wound Drains
- Surgical Equipment/Accessories
- Surgical Instruments
- Bandage Scissors
- Bandage Scissors N/S
- Bandage Scissors Strl
- Biopsy Punches
- Biopsy Punches N/S
- Blade Remover
- Cervical Dilators
- Cervical Dilators N/S
- Conventional Handles
- Conventional Handles N/S
- Conventional Scalpel Blades
- Cpr Emergency Kits
- Dental Hand Instruments
- Dental Hand Instruments N/S
- Dissecting Scissors
- Ear Curets
- Ear Curets N/S
- Ear Piercing Instruments
- Ear Piercing Instruments N/S
- Ear Syringes
- Ear Syringes N/S
- Ent Forceps
- Ent Forceps N/S
- Eye, Scissors: Surgical Instruments
- General Instr, Tools, Laboratory N/S
- General Instruments
- General Instruments N/S
- General Instruments, Tools, Laboratory
- Gravity Enteral Feeding Kits
- Gravity Enteral Feeding Kits N/S
- Implantation Instruments, Orthopedic
- Implantation Instruments, Orthopedic N/S
- Implnts-Screw-Burrs-Saw
- Instrument Sets
- Instrument Sets N/S
- Knives/Blades, Plaster: Orthopedic
- Magnifiers: Surgical Instruments
- Markers, X-Ray Radiological
- Mastoid Retractors
- Mastoid Retractors N/S
- Miscellaneous Endoscopic Supplies
- Miscellaneous Needles & Syringes
- Nasal Specula
- Nasal Specula N/S
- Nasal Specula Strl
- Needle Counters
- Neurological Hammers
- Neurological Hammers N/S
- Operating Room Scissors
- Ophthalmic Occluders
- Other Clamps
- Other Clamps Strl
- Other Surgical Scissors
- Other Surgical Scissors N/S
- Parts, Surgical Instruments
- Percussion Hammers
- Percussion Hammers N/S
- Pessaries
- Pessaries N/S
- Pinwheels: Surgical Instruments
- Pinwheels: Surgical Instruments N/S
- Protectors, Surgical Instruments
- Razors, Skin Prep: Surgical Instruments
- Recapping Devices Syr/Ndl
- Safety Handles N/S
- Sets/Units, Cryosurgery, Surgical Instru
- Skin, Markers: Surgical Instruments
- Specialty Scalpel Blades
- Specimen Forceps
- Splinter Forceps
- Splinter Forceps N/S
- Sponge Counters
- Surgical Access, Other Instrumentation
- Surgical Accessories, Miscellaneous Surg
- Suture Scissors
- Suture Scissors N/S
- Tip Caps, Syringe
- Tip Caps, Syringe N/S
- Tips, Suction
- Tips, Suction N/S
- Tonsil Scissors
- Tuning Forks
- Tuning Forks N/S
- Tweezers: Surgical Instruments
- Tweezers: Surgical Instruments N/S
- Uterine Aspirators N/S
- Utility Forceps
- Utility Forceps N/S
- Table Paper
- Tapes/Wraps
- Applicators, Tubular
- Athletic/Elastic
- Awc
- Bandages
- Bandages, Other Pressure Relief
- Compression Bandages
- Conforming Bandages
- Dressings
- Elastic Bandages
- First Aid Kits
- General Purpose Adhesive Strips
- General Purpose Adhesive Tapes
- Hypoallergenic Adhesive Tapes
- Nonsterile Gauze
- Other Bandages
- Other Tapes/Wraps, Non-Adherent/Burn/Com
- Other Tapes/Wraps, Other
- Other Tapes/Wraps, Other – Bandage/Dress
- Other Tapes/Wraps, Tapes/Band-Aids
- Retainer Bandages
- Self-Adherent Bandages
- Tubular Support Bandages
- Textiles
- Aprons, Staff
- Bath/Hand Towels
- Bedding, Patient, Disposable/Reusable
- Bedspreads
- Bibs, Reusable
- Blanket Reusable
- Dining/Kitchen Linen Reusable
- Drape/Sheets, Nonbedding, Disposable
- Drapes Equipment Covers
- Equipment/Instruments, Covers/Drapes
- Exam Table Headrest Covers
- Infant Scale Liners, Pediatric Supp
- Laundry Bags Reusable
- Mattress Covers Impervious
- Mattress Covers, Textiles
- Other Pillows
- Patient Pillows
- Patient Woven Goods, Other Metal/Plastic
- Patient Woven Goods, Rehabilitation Wear
- Pillow Covers
- Pillows Reusable
- Practice Woven Goods, Other Metal/Plasti
- Professional Disposable Towels
- Rehabilitation Wear
- Robes, Patient
- Sheets/Pillowcases Reusable
- Shroud Kits
- Slippers
- Surgical Equipment Drapes
- Table Covers/Drapes, Surgical
- Textiles, Bedspreads
- Textiles, Blanket Reusable
- Textiles, Pillows Reusable
- Textiles, Textiles
- Towels: Surgical, Disposable
- Towels/Washcloths Reusable
- Warm-Up Wear, Staff
- Therapy Massagers
- Training Equipment & Supplies
- Tympanic/Ear Thermometer Probe Covers
- Uncategorized
- Underpads, Pads & Liners
- Urinalysis Reagents
- Urology
- Access-Irr Tray/Bulb/Syrng
- Catheter Straps/Holders
- Catheters/Foley/Retent/Irrig
- Conventional Hypo Syringes
- Drainage Bags/Tubes
- Facial And Eye Protection
- Irrigtrays/Bulbs/Syringes, Other
- Irrigtrays/Bulbs/Syringes, Tray/Kit, Art
- Male External
- Other Urological Supplies
- Other Urological, Foams
- Straight Intermittent
- Urethral/Foley Trays
- Utensils/Pt Stay/Plas – Disp
- Wound Closure
TEST KIT, INFLUENZA A & B CONSULT CLIA WAIVED (25TEST/KIT)
RESTRICTED - NOT ON FORMULARY
Rapid Test Kit McKesson Consult™ Infectious Disease Immunoassay Influenza A + B Nasal Swab / Nasopharyngeal Wash / Nasopharyngeal Aspirate Sample 25 Tests CLIA Waived
Item No.
1076728
Categories Flu - Visually Read, Lab-Respiratory Testing
| Units of Measurement | |
|---|---|
| Manufacturer | McKesson MedSurg |
| UNSPSC | 41116144 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_MGM181_TEST KIT_INFLUENZA_A_&_B_CLIAWAIVED_25TEST_KIT.pdf |
| SDS | SDS_MGM181_TEST KIT_INFLUENZA_A_&_B_CLIAWAIVED_25TEST_KIT.pdf |
| Product Spec Sheet | Consult_flu_kits_tear_sheet_2018-11.pdf |
| Procedure Card | 181-36025_procedure_ card_ 2019-08.pdf |
| Instructions for Use | FLU_Instructions_for_Use_181-36025-03-2020.pdf |
| Annual Reactivity Testing Results | P-52760-C_McKesson_Flu_2018-2019-2020_Annual_Reactivity_Testing_Results_appendix-1.pdf |
| Procedure Sheet | CLSI_McKesson_Consult_Influenza_A&B_Procedure.pdf |
Related products
-
Covid - Rapids
TEST KIT, SARS ANTIGEN QUICKVUE PROFESSIONAL USE (25/KT)
-
Strep - Molecular - Waived
TEST KIT, STREP A 2 ALERE (24/KT)
-
Rsv - Visually Read
TEST KT, QKVU RSV 20 CLWV (20/KT 12KT/CS)
-
Rsv - Molecular - Waived
TEST KIT, ALERE I RSV (24/KT)