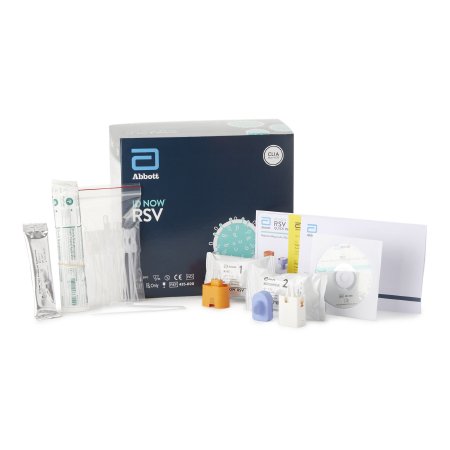
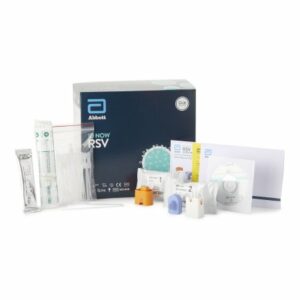
TEST KIT, RSV ID NOW (24/KT)
RESTRICTED - NOT ON FORMULARY
ID NOW™ RSV detects the RSV virus in nasopharyngeal (NP) swab samples using Alere’s proprietary Molecular In Minutes™ isothermal nucleic acid amplification technology (iNAT)
ID NOW™ i RSV is a rapid (less than 15 minutes), instrument-based isothermal test for the qualitative detection of RSV A and RSV B from nasopharyngeal swabs and nasopharyngeal swabs eluted in viral transport media
It is comprised of a Sample Receiver, containing elution buffer, a Test Base, comprising two sealed reaction tubes, each containing a lyophilized pellet, a Transfer Cartridge for transfer of the eluted sample to the Test Base
The reaction tubes in the Test Base contain the reagents required for amplification of RSV A and RSV B, respectively, as well as an internal control
Intended for use as an aid in the diagnosis of RSV in children <18 years and adults ≥60 years in conjunction with clinical and epidemiological risk factors
Product Specification Sheet
| Units of Measurement | |
|---|---|
| Manufacturer | ABBOTT RAPID DX |
| UNSPSC | 41116144 |
| Product Specification Sheet | https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_WAMPL_TEST_KIT_ALERE_IRSV_24KT.pdf |
| SDS | SDS_WAMPL_TEST_KIT_ALERE_IRSV_24KT.pdf |
| ID Now RSV Test Package Insert | ID_Now_RSV_Package_Insert.pdf |
Related Products
Related products
-
Covid - Rapids
TEST KIT, COVID-19 QUICKVUE AT-HOME OTC (25TEST/KT 10KT/CS)
This product has multiple variants. The options may be chosen on the product page