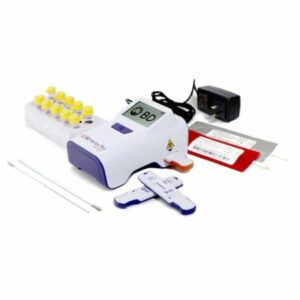
ID NOW™ COVID-19 control kit (12 Positive, 12 Negative)
External Positive and Negative Control swabs should be tested following the Run QC Test instructions on the ID NOW Instrument
For Use Under an Emergency Use Authorization (EUA) Only: https://www.fda.gov/medical-devices/emergency-situations-medical-devices/emergency-use-authorizations
| Units of Measurement | |
|---|---|
| Manufacturer | ABBOTT RAPID DX |
| UNSPSC | 41116128 |
| Product Specification Sheet | |
| SDS | SDS_WAMPL_CONTROL_KIT_COVID_19_ID_NOW_12POS_12NEG_KT_D_S.PDF |
| ID NOW COVID19 EUA | ID_NOW_COVID19_EUA.pdf |
| ID NOW COVID19 Product Sheet | ID_NOW_COVID19_Product_Sheet.pdf |
| COVID19 Health Care Providers Fact Sheet | COVID19_Fact_Sheet_Healthcare_Providers.pdf |
| COVID19 Patient Fact Sheet | COVID19_Fact_Sheet_Patients |
| ID NOW COVID19 Product Insert | ID_NOW_COVID19_Product_Insert.pdf |
Related Products
Related products
-
Lab-Respiratory Testing
TEST KIT, QUICKVUE STREP DIP STICK (50/KT 12KT/CS)
This product has multiple variants. The options may be chosen on the product page -
Covid - Rapids
TEST KIT, COVID-19 QUICKVUE AT-HOME OTC (2/BX 45BX/CS)
This product has multiple variants. The options may be chosen on the product page